Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes
- PMID: 11402073
- PMCID: PMC2192029
- DOI: 10.1083/jcb.153.6.1315
Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes
Abstract
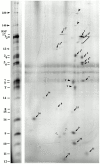
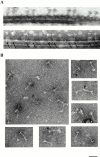
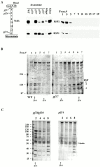
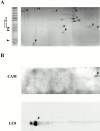
Genetic and in vitro analyses have revealed that radial spokes play a crucial role in regulation of ciliary and flagellar motility, including control of waveform. However, the mechanisms of regulation are not understood. Here, we developed a novel procedure to isolate intact radial spokes as a step toward understanding the mechanism by which these complexes regulate dynein activity. The isolated radial spokes sediment as 20S complexes that are the size and shape of radial spokes. Extracted radial spokes rescue radial spoke structure when reconstituted with isolated axonemes derived from the radial spoke mutant pf14. Isolated radial spokes are composed of the 17 previously defined spoke proteins as well as at least five additional proteins including calmodulin and the ubiquitous dynein light chain LC8. Analyses of flagellar mutants and chemical cross-linking studies demonstrated calmodulin and LC8 form a complex located in the radial spoke stalk. We postulate that calmodulin, located in the radial spoke stalk, plays a role in calcium control of flagellar bending.
Figures
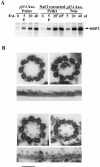
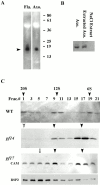
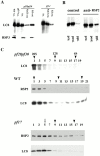

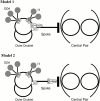
Similar articles
-
Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas.J Cell Sci. 1996 Jul;109 ( Pt 7):1899-907. doi: 10.1242/jcs.109.7.1899. J Cell Sci. 1996. PMID: 8832412
-
Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase.J Cell Biol. 1994 Dec;127(6 Pt 1):1683-92. doi: 10.1083/jcb.127.6.1683. J Cell Biol. 1994. PMID: 7798320 Free PMC article.
-
Flagellar radial spokes contain a Ca2+-stimulated nucleoside diphosphate kinase.Mol Biol Cell. 2004 Aug;15(8):3891-902. doi: 10.1091/mbc.e04-04-0352. Epub 2004 Jun 11. Mol Biol Cell. 2004. PMID: 15194815 Free PMC article.
-
Ciliary Motility: Regulation of Axonemal Dynein Motors.Cold Spring Harb Perspect Biol. 2017 Aug 1;9(8):a018325. doi: 10.1101/cshperspect.a018325. Cold Spring Harb Perspect Biol. 2017. PMID: 28765157 Free PMC article. Review.
-
Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation.Cell Motil Cytoskeleton. 2007 Aug;64(8):569-79. doi: 10.1002/cm.20211. Cell Motil Cytoskeleton. 2007. PMID: 17549744 Review.
Cited by
-
The CSC proteins FAP61 and FAP251 build the basal substructures of radial spoke 3 in cilia.Mol Biol Cell. 2015 Apr 15;26(8):1463-75. doi: 10.1091/mbc.E14-11-1545. Epub 2015 Feb 18. Mol Biol Cell. 2015. PMID: 25694453 Free PMC article.
-
Analysis of cargo transport by IFT and GFP imaging of IFT in Chlamydomonas.Methods Cell Biol. 2009;93:111-9. doi: 10.1016/S0091-679X(08)93006-5. Epub 2009 Dec 4. Methods Cell Biol. 2009. PMID: 20409814 Free PMC article.
-
LRRC23 is a conserved component of the radial spoke that is necessary for sperm motility and male fertility in mice.J Cell Sci. 2021 Oct 15;134(20):jcs259381. doi: 10.1242/jcs.259381. Epub 2021 Oct 21. J Cell Sci. 2021. PMID: 34585727 Free PMC article.
-
Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system.Eukaryot Cell. 2006 Apr;5(4):696-711. doi: 10.1128/EC.5.4.696-711.2006. Eukaryot Cell. 2006. PMID: 16607017 Free PMC article.
-
Regulation of dynein-driven microtubule sliding by the axonemal protein kinase CK1 in Chlamydomonas flagella.J Cell Biol. 2009 Sep 21;186(6):817-24. doi: 10.1083/jcb.200906168. Epub 2009 Sep 14. J Cell Biol. 2009. PMID: 19752022 Free PMC article.
References
-
- Bannai H., Yoshima M., Takahashi K., Shingyoji C. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J. Cell Sci. 2000;113:831–839. - PubMed
-
- Benashski S.E., Harrison A., Patel-King R.S., King S.M. Dimerization of the highly conserved light chain shared by dynein and myosin V. J. Biol. Chem. 1997;272:20929–20935. - PubMed
-
- Brokaw C.J. Regulation of sperm flagellar motility by calcium and cAMP-dependent phosphorylation. J. Cell. Biochem. 1987;35:175–184. - PubMed
-
- Brokaw C.J. Control of flagellar bendinga new agenda based on dynein diversity. Cell Motil. Cytoskel. 1994;28:199–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

