Alteration of auxin polar transport in the Arabidopsis ifl1 mutants
- PMID: 11402186
- PMCID: PMC111148
- DOI: 10.1104/pp.126.2.549
Alteration of auxin polar transport in the Arabidopsis ifl1 mutants
Abstract
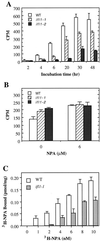
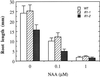
The INTERFASCICULAR FIBERLESS/REVOLUTA (IFL1/REV) gene is essential for the normal differentiation of interfascicular fibers and secondary xylem in the inflorescence stems of Arabidopsis. It has been proposed that IFL1/REV influences auxin polar flow or the transduction of auxin signal, which is required for fiber and vascular differentiation. Assay of auxin polar transport showed that the ifl1 mutations dramatically reduced auxin polar flow along the inflorescence stems and in the hypocotyls. The null mutant allele ifl1-2 was accompanied by a significant decrease in the expression level of two putative auxin efflux carriers. The ifl1 mutants remained sensitive to auxin and an auxin transport inhibitor. The ifl1-2 mutant exhibited visible phenotypes associated with defects in auxin polar transport such as pin-like inflorescence, reduced numbers of cauline branches, reduced numbers of secondary rosette inflorescence, and dark green leaves with delayed senescence. The visible phenotypes displayed by the ifl1 mutants could be mimicked by treatment of wild-type plants with an auxin polar transport inhibitor. In addition, the auxin polar transport inhibitor altered the normal differentiation of interfascicular fibers in the inflorescence stems of wild-type Arabidopsis. Taken together, these results suggest a correlation between the reduced auxin polar transport and the alteration of cell differentiation and morphology in the ifl1 mutants.
Figures









References
-
- Aloni R. Polarity of induction and pattern of primary phloem fiber differentiation in Coleus. Am J Bot. 1976;63:877–889.
-
- Aloni R. Source of induction and sites of primary phloem fiber differentiation in Coleus blumei. Ann Bot. 1978;42:1261–1269.
-
- Aloni R. Differentiation of vascular tissues. Annu Rev Plant Physiol. 1987;38:179–204.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

