The Arabidopsis embryo mutant schlepperless has a defect in the chaperonin-60alpha gene
- PMID: 11402200
- PMCID: PMC111162
- DOI: 10.1104/pp.126.2.717
The Arabidopsis embryo mutant schlepperless has a defect in the chaperonin-60alpha gene
Abstract
We identified a T-DNA-generated mutation in the chaperonin-60alpha gene of Arabidopsis that produces a defect in embryo development. The mutation, termed schlepperless (slp), causes retardation of embryo development before the heart stage, even though embryo morphology remains normal. Beyond the heart stage, the slp mutation results in defective embryos with highly reduced cotyledons. slp embryos exhibit a normal apical-basal pattern and radial tissue organization, but they are morphologically retarded. Even though slp embryos are competent to transcribe two late-maturation gene markers, this competence is acquired more slowly as compared with wild-type embryos. slp embryos also exhibit a defect in plastid development-they remain white during maturation in planta and in culture. Hence, the overall developmental phenotype of the slp mutant reflects a lesion in the chloroplast that affects embryo development. The slp phenotype highlights the importance of the chaperonin-60alpha protein for chloroplast development and subsequently for the proper development of the plant embryo and seedling.
Figures




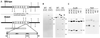


References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocol in Molecular Biology. New York: Greene Publishing and Wiley-Interscience; 1992.
-
- Behringer FJ, Medford JI. A plasmid rescue technique for the recovery of plant DNA disrupted by T-DNA insertion. Plant Mol Biol Rep. 1992;10:190–198.
-
- Berleth T, Jürgens G. The role of monopteros in organizing the basal body region of the Arabidopsis embryo. Development. 1993;118:575–587.
-
- Castle LA, Errampalli D, Aterton TL, Franzmann LH, Yoon ES, Meinke DW. Genetic and molecular characterization of embryonic mutants identified following seed transformation in Arabidopsis. Mol Gen Genet. 1993;241:504–514. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

