Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed arabidopsis
- PMID: 11402204
- PMCID: PMC111166
- DOI: 10.1104/pp.126.2.759
Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed arabidopsis
Abstract
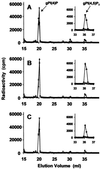
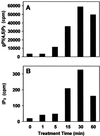
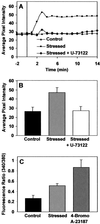
The phosphoinositide phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P(2)] is a key signaling molecule in animal cells. It can be hydrolyzed to release 1,2-diacyglycerol and inositol 1,4,5-trisphosphate (IP(3)), which in animal cells lead to protein kinase C activation and cellular calcium mobilization, respectively. In addition to its critical roles in constitutive and regulated secretion of proteins, PtdIns(4,5)P(2) binds to proteins that modify cytoskeletal architecture and phospholipid constituents. Herein, we report that Arabidopsis plants grown in liquid media rapidly increase PtdIns(4,5)P(2) synthesis in response to treatment with sodium chloride, potassium chloride, and sorbitol. These results demonstrate that when challenged with salinity and osmotic stress, terrestrial plants respond differently than algae, yeasts, and animal cells that accumulate different species of phosphoinositides. We also show data demonstrating that whole-plant IP(3) levels increase significantly within 1 min of stress initiation, and that IP(3) levels continue to increase for more than 30 min during stress application. Furthermore, using the calcium indicators Fura-2 and Fluo-3 we show that root intracellular calcium concentrations increase in response to stress treatments. Taken together, these results suggest that in response to salt and osmotic stress, Arabidopsis uses a signaling pathway in which a small but significant portion of PtdIns(4,5)P(2) is hydrolyzed to IP(3). The accumulation of IP(3) occurs during a time frame similar to that observed for stress-induced calcium mobilization. These data also suggest that the majority of the PtdIns(4,5)P(2) synthesized in response to salt and osmotic stress may be utilized for cellular signaling events distinct from the canonical IP(3) signaling pathway.
Figures
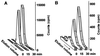

Similar articles
-
Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells.J Biol Chem. 1999 Dec 31;274(53):38232-40. doi: 10.1074/jbc.274.53.38232. J Biol Chem. 1999. PMID: 10608898
-
Sustained elevation in inositol 1,4,5-trisphosphate results in inhibition of phosphatidylinositol transfer protein activity and chronic depletion of the agonist-sensitive phosphoinositide pool.J Cell Sci. 2000 Jul;113 ( Pt 14):2631-8. doi: 10.1242/jcs.113.14.2631. J Cell Sci. 2000. PMID: 10862720
-
Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway.Plant Physiol. 2005 Jun;138(2):686-700. doi: 10.1104/pp.105.061317. Epub 2005 May 27. Plant Physiol. 2005. PMID: 15923324 Free PMC article.
-
Phosphoinositide 3-kinases-a historical perspective.Subcell Biochem. 2012;58:95-110. doi: 10.1007/978-94-007-3012-0_4. Subcell Biochem. 2012. PMID: 22403075 Review.
-
Phosphoinositide phosphatases: just as important as the kinases.Subcell Biochem. 2012;58:215-79. doi: 10.1007/978-94-007-3012-0_7. Subcell Biochem. 2012. PMID: 22403078 Review.
Cited by
-
Isolation and functional characterization of a cold responsive phosphatidylinositol transfer-associated protein, ZmSEC14p, from maize (Zea may L.).Plant Cell Rep. 2016 Aug;35(8):1671-86. doi: 10.1007/s00299-016-1980-4. Epub 2016 Apr 9. Plant Cell Rep. 2016. PMID: 27061906
-
Salt tolerance.Arabidopsis Book. 2002;1:e0048. doi: 10.1199/tab.0048. Epub 2002 Sep 30. Arabidopsis Book. 2002. PMID: 22303210 Free PMC article.
-
Calcium at the crossroads of signaling.Plant Cell. 2002;14 Suppl(Suppl):S401-17. doi: 10.1105/tpc.002899. Plant Cell. 2002. PMID: 12045291 Free PMC article. Review. No abstract available.
-
A dibasic amino acid pair conserved in the activation loop directs plasma membrane localization and is necessary for activity of plant type I/II phosphatidylinositol phosphate kinase.Plant Physiol. 2010 Jul;153(3):1004-15. doi: 10.1104/pp.109.152686. Epub 2010 Apr 28. Plant Physiol. 2010. PMID: 20427464 Free PMC article.
-
Phosphoinositides in plant-pathogen interaction: trends and perspectives.Stress Biol. 2023 Apr 6;3(1):4. doi: 10.1007/s44154-023-00082-5. Stress Biol. 2023. PMID: 37676371 Free PMC article. Review.
References
-
- Alexandre J, Lassalles JP, Kado RT. Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5 trisphosphate. Nature. 1990;343:567–570.
-
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

