Genetic control of natural variation in Arabidopsis glucosinolate accumulation
- PMID: 11402209
- PMCID: PMC111171
- DOI: 10.1104/pp.126.2.811
Genetic control of natural variation in Arabidopsis glucosinolate accumulation
Abstract
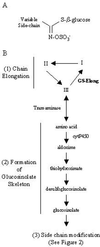
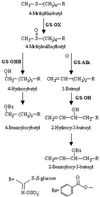
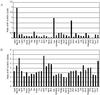
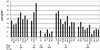
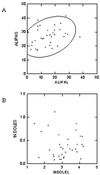
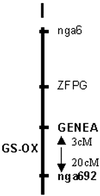
Glucosinolates are biologically active secondary metabolites of the Brassicaceae and related plant families that influence plant/insect interactions. Specific glucosinolates can act as feeding deterrents or stimulants, depending upon the insect species. Hence, natural selection might favor the presence of diverse glucosinolate profiles within a given species. We determined quantitative and qualitative variation in glucosinolates in the leaves and seeds of 39 Arabidopsis ecotypes. We identified 34 different glucosinolates, of which the majority are chain-elongated compounds derived from methionine. Polymorphism at only five loci was sufficient to generate 14 qualitatitvely different leaf glucosinolate profiles. Thus, there appears to be a modular genetic system regulating glucosinolate profiles in Arabidopsis. This system allows the rapid generation of new glucosinolate combinations in response to changing herbivory or other selective pressures. In addition to the qualitative variation in glucosinolate profiles, we found a nearly 20-fold difference in the quantity of total aliphatic glucosinolates and were able to identify a single locus that controls nearly three-quarters of this variation.
Figures

References
-
- Bartlet E, Parsons D, Williams I, Clark S. The influence of glucosinolates and sugars on feeding by the cabbage stem flea beetle, Psylliodes chrysocephala. Entomol Exp Appl. 1994;73:77–83.
-
- Chisholm MD, Wetter LR. Biosynthesis of mustard oil glucosides: IV. The administration of methionine-14C and related compounds to horseradish. Can J Biochem. 1964;42:1033–1040. - PubMed
-
- Daxenbichler ME, Spencer GF, Carlson DG, Rose GB, Brinkler AM, Powell RG. Glucosinolate composition of seeds from 297 species of wild plants. Phytochemistry. 1991;30:2623–2638.
-
- de Quiros HC, Magrath R, McCallum D, Kroymann J, Schnabelrauch D, Mitchell-Olds T, Mithen R. α-Keto acid elongation and glucosinolate biosynthesis in Arabidopsis thaliana. Theor Appl Genet. 2000;101:429–437.
-
- Giamoustaris A, Mithen R. The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica Napus Ssp Oleifera) on its interaction with specialist and generalist pests. Ann Appl Biol. 1995;126:347–363.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

