Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight
- PMID: 11402213
- PMCID: PMC111175
- DOI: 10.1104/pp.126.2.861
Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight
Abstract
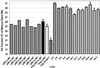
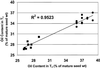
We recently reported the cloning and characterization of an Arabidopsis (ecotype Columbia) diacylglycerol acyltransferase cDNA (Zou et al., 1999) and found that in Arabidopsis mutant line AS11, an ethyl methanesulfonate-induced mutation at a locus on chromosome II designated as Tag1 consists of a 147-bp insertion in the DNA, which results in a repeat of the 81-bp exon 2 in the Tag1 cDNA. This insertion mutation is correlated with an altered seed fatty acid composition, reduced diacylglycerol acyltransferase (DGAT; EC 2.3.1.20) activity, reduced seed triacylglycerol content, and delayed seed development in the AS11 mutant. The effect of the insertion mutation on microsomal acyl-coenzyme A-dependent DGAT is examined with respect to DGAT activity and its substrate specificity in the AS11 mutant relative to wild type. We demonstrate that transformation of mutant AS11 with a single copy of the wild-type Tag1 DGAT cDNA can complement the fatty acid and reduced oil phenotype of mutant AS11. More importantly, we show for the first time that seed-specific over-expression of the DGAT cDNA in wild-type Arabidopsis enhances oil deposition and average seed weight, which are correlated with DGAT transcript levels. The DGAT activity in developing seed of transgenic lines was enhanced by 10% to 70%. Thus, the current study confirms the important role of DGAT in regulating the quantity of seed triacylglycerols and the sink size in developing seeds.
Figures








References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Barron EJ, Stumpf PK. The biosynthesis of triglycerides by avocado mesocarp enzymes. Biochem Biophys Acta. 1962;60:329–337. - PubMed
-
- Bernerth R, Frentzen M. Utilization of erucoyl-CoA by acyltransferases from developing seeds of Brassica napus(L.) involved in triacylglycerol biosynthesis. Plant Sci. 1990;67:21–28.
-
- Bouvier-Navé P, Benveniste P, Oelkers P, Sturley SL, Schaller H. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur J Biochem. 2000;267:85–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

