BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses
- PMID: 11404410
- PMCID: PMC2806848
- DOI: 10.1523/JNEUROSCI.21-12-04249.2001
BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses
Abstract
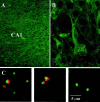
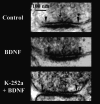
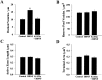
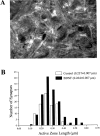
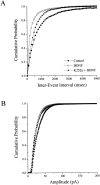
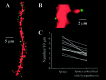
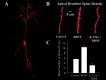
Brain-derived neurotrophic factor (BDNF) is emerging as a key mediator of activity-dependent modifications of synaptic strength in the CNS. We investigated the hypothesis that BDNF enhances quantal neurotransmitter release by modulating the distribution of synaptic vesicles within presynaptic terminals using organotypic slice cultures of postnatal rat hippocampus. BDNF specifically increased the number of docked vesicles at the active zone of excitatory synapses on CA1 dendritic spines, with only a small increase in active zone size. In agreement with the hypothesis that an increased docked vesicle density enhances quantal neurotransmitter release, BDNF increased the frequency, but not the amplitude, of AMPA receptor-mediated miniature EPSCs (mEPSCs) recorded from CA1 pyramidal neurons in hippocampal slices. Synapse number, independently estimated from dendritic spine density and electron microscopy measurements, was also increased after BDNF treatment, indicating that the actions of BNDF on mEPSC frequency can be partially attributed to an increased synaptic density. Our results further suggest that all these actions were mediated via tyrosine kinase B (TrkB) receptor activation, established by inhibition of plasma membrane tyrosine kinases with K-252a. These results provide additional evidence of a fundamental role of the BDNF-TrkB signaling cascade in synaptic transmission, as well as in cellular models of hippocampus-dependent learning and memory.
Figures

Similar articles
-
Reduced number of functional glutamatergic synapses in hippocampal neurons overexpressing full-length TrkB receptors.J Neurosci Res. 2001 Nov 1;66(3):327-36. doi: 10.1002/jnr.10007. J Neurosci Res. 2001. PMID: 11746350
-
Giant miniature EPSCs at the hippocampal mossy fiber to CA3 pyramidal cell synapse are monoquantal.J Neurophysiol. 2002 Jan;87(1):15-29. doi: 10.1152/jn.00394.2001. J Neurophysiol. 2002. PMID: 11784726
-
Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission.J Neurophysiol. 2008 Dec;100(6):3175-84. doi: 10.1152/jn.90880.2008. Epub 2008 Oct 15. J Neurophysiol. 2008. PMID: 18922945 Free PMC article.
-
The role of neurotrophins in neurotransmitter release.Neuroscientist. 2002 Dec;8(6):524-31. doi: 10.1177/1073858402238511. Neuroscientist. 2002. PMID: 12467374 Free PMC article. Review.
-
New insights into the role of brain-derived neurotrophic factor in synaptic plasticity.Mol Cell Neurosci. 2009 Oct;42(2):81-9. doi: 10.1016/j.mcn.2009.06.009. Epub 2009 Jul 3. Mol Cell Neurosci. 2009. PMID: 19577647 Free PMC article. Review.
Cited by
-
Developmental changes in brain-derived neurotrophic factor-mediated modulations of synaptic activities in the pontine Kölliker-Fuse nucleus of the rat.J Physiol. 2007 Aug 15;583(Pt 1):315-27. doi: 10.1113/jphysiol.2007.134726. Epub 2007 Jun 14. J Physiol. 2007. PMID: 17569735 Free PMC article.
-
ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons.Learn Mem. 2004 Mar-Apr;11(2):172-8. doi: 10.1101/lm.67804. Learn Mem. 2004. PMID: 15054132 Free PMC article.
-
Recovery of stress response coincides with responsiveness to voluntary exercise after traumatic brain injury.J Neurotrauma. 2014 Apr 1;31(7):674-82. doi: 10.1089/neu.2013.3151. Epub 2013 Dec 19. J Neurotrauma. 2014. PMID: 24151829 Free PMC article.
-
Characterization of early pathogenesis in the SOD1(G93A) mouse model of ALS: part I, background and methods.Brain Behav. 2013 Jul;3(4):335-50. doi: 10.1002/brb3.143. Epub 2013 Jun 11. Brain Behav. 2013. PMID: 24381807 Free PMC article.
-
Effects of General Anesthetics on Synaptic Transmission and Plasticity.Curr Neuropharmacol. 2022;20(1):27-54. doi: 10.2174/1570159X19666210803105232. Curr Neuropharmacol. 2022. PMID: 34344292 Free PMC article. Review.
References
-
- Aoki C, Wu K, Elste A, Len GW, Lin SY, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
