Phenserine regulates translation of beta -amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development
- PMID: 11404470
- PMCID: PMC34715
- DOI: 10.1073/pnas.131152998
Phenserine regulates translation of beta -amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development
Abstract
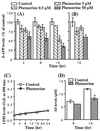
The reduction in levels of the potentially toxic amyloid-beta peptide (Abeta) has emerged as one of the most important therapeutic goals in Alzheimer's disease. Key targets for this goal are factors that affect the expression and processing of the Abeta precursor protein (betaAPP). Earlier reports from our laboratory have shown that a novel cholinesterase inhibitor, phenserine, reduces betaAPP levels in vivo. Herein, we studied the mechanism of phenserine's actions to define the regulatory elements in betaAPP processing. Phenserine treatment resulted in decreased secretion of soluble betaAPP and Abeta into the conditioned media of human neuroblastoma cells without cellular toxicity. The regulation of betaAPP protein expression by phenserine was posttranscriptional as it suppressed betaAPP protein expression without altering betaAPP mRNA levels. However, phenserine's action was neither mediated through classical receptor signaling pathways, involving extracellular signal-regulated kinase or phosphatidylinositol 3-kinase activation, nor was it associated with the anticholinesterase activity of the drug. Furthermore, phenserine reduced expression of a chloramphenicol acetyltransferase reporter fused to the 5'-mRNA leader sequence of betaAPP without altering expression of a control chloramphenicol acetyltransferase reporter. These studies suggest that phenserine reduces Abeta levels by regulating betaAPP translation via the recently described iron regulatory element in the 5'-untranslated region of betaAPP mRNA, which has been shown previously to be up-regulated in the presence of interleukin-1. This study identifies an approach for the regulation of betaAPP expression that can result in a substantial reduction in the level of Abeta.
Figures



References
-
- Selkoe D J. Science. 1997;275:630–631. - PubMed
-
- Roberson M, Harrell L. Brain Res Rev. 1997;25:50–69. - PubMed
-
- Checler F. J Neurochem. 1995;65:1431–1444. - PubMed
-
- Hussain I, Powell D, Howlett D, Tew D, Meek T, Chapman C, Gloger I, Murphy K, Southan C, Ryan D, et al. Mol Cell Neurosci. 1999;14:419–427. - PubMed
-
- Sinha S, Anderson J P, Barbour R, Basi G, Caccavello R, Davis D, Dovey H, Frigon N, Hong J, et al. Nature (London) 1999;402:537–540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

