Crystal structure of an anticoagulant protein in complex with the Gla domain of factor X
- PMID: 11404471
- PMCID: PMC34651
- DOI: 10.1073/pnas.131179698
Crystal structure of an anticoagulant protein in complex with the Gla domain of factor X
Abstract
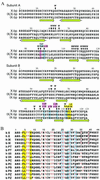
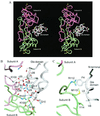
The gamma-carboxyglutamic acid (Gla) domain of blood coagulation factors is responsible for Ca2+-dependent phospholipid membrane binding. Factor X-binding protein (X-bp), an anticoagulant protein from snake venom, specifically binds to the Gla domain of factor X. The crystal structure of X-bp in complex with the Gla domain peptide of factor X at 2.3-A resolution showed that the anticoagulation is based on the fact that two patches of the Gla domain essential for membrane binding are buried in the complex formation. The Gla domain thus is expected to be a new target of anticoagulant drugs, and X-bp provides a basis for designing them. This structure also provides a membrane-bound model of factor X.
Figures
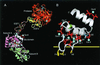
References
-
- Mann K G. Thromb Haemostasis. 1999;82:165–174. - PubMed
-
- Leung D, Abbenante G, Fairlie D P. J Med Chem. 2000;43:305–341. - PubMed
-
- Dennis M S, Elgenbrot C, Skelton N J, Ultsch M H, Santell L, Dwyer M A, O'Connell M P, Lazarus R A. Nature (London) 2000;404:465–470. - PubMed
-
- Atoda H, Hyuga M, Morita T. J Biol Chem. 1991;266:14903–14911. - PubMed
-
- Atoda H, Ishikawa M, Yoshihara E, Sekiya F, Morita T. J Biochem. 1995;118:965–973. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

