EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG
- PMID: 11406581
- PMCID: PMC150189
- DOI: 10.1093/emboj/20.12.3046
EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG
Abstract
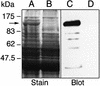
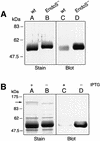
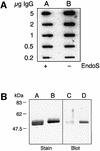
Streptococcus pyogenes is an important human pathogen that selectively interacts with proteins involved in the humoral defense system, such as immunoglobulins and complement factors. In this report we show that S.pyogenes has the ability to hydrolyze the chitobiose core of the asparagine-linked glycan on immuno globulin G (IgG) when bacteria are grown in the presence of human plasma. This activity is associated with the secretion of a novel 108 kDa protein denoted EndoS. EndoS has endoglycosidase activity on purified soluble IgG as well as IgG bound to the bacterial surface. EndoS is required for the activity on IgG, as an isogenic EndoS mutant could not hydrolyze the glycan on IgG. In addition, we show that the secreted streptococcal cysteine proteinase SpeB cleaves IgG in the hinge region in a papain-like manner. This is the first example of an endoglycosidase produced by a bacterial pathogen that selectively hydrolyzes human IgG, and reveals a novel mechanism which may contribute to S.pyogenes pathogenesis.
Figures
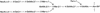






References
-
- Åkesson P., Cooney,J., Kishimoto,F. and Björck,L. (1990) Protein H—a novel IgG binding bacterial protein. Mol. Immunol., 27, 523–531. - PubMed
-
- Alexander S. and Elder,J.H. (1989) Endoglycosidases from Flavo bacterium meningosepticum: application to biological problems. Methods Enzymol., 179, 505–518. - PubMed
-
- Altschul S.F., Gish,W., Miller,W., Myers,E.W. and Lipman,D.J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. - PubMed
-
- Axford J.S., Mackenzie,L., Lydyard,P.M., Hay,F.C., Isenberg,D.A. and Roitt,I.M. (1987) Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet, 2, 1486–1488. - PubMed
-
- Baenziger J. and Kornfeld,S. (1974) Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation and structure of the asparagine-linked oligosaccharide units. J. Biol. Chem., 249, 7260–7269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

