Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation
- PMID: 11406587
- PMCID: PMC150191
- DOI: 10.1093/emboj/20.12.3101
Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation
Abstract
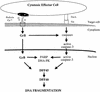
The protease granzyme B (GrB) plays a key role in the cytocidal activity during cytotoxic T lymphocyte (CTL)-mediated programmed cell death. Multiple caspases have been identified as direct substrates for GrB, suggesting that the activation of caspases constitutes an important event during CTL-induced cell death. However, recent studies have provided evidence for caspase-independent pathway(s) during CTL-mediated apoptosis. In this study, we demonstrate caspase-independent and direct cleavage of the 45 kDa unit of DNA fragmentation factor (DFF45) by GrB both in vitro and in vivo. Using a novel and selective caspase-3 inhibitor, we show the ability of GrB to process DFF45 directly and mediate DNA fragmentation in the absence of caspase-3 activity. Furthermore, studies with DFF45 mutants reveal that both caspase-3 and GrB share a common cleavage site, which is necessary and sufficient to induce DNA fragmentation in target cells during apoptosis. Together, our data suggest that CTLs possess alternative mechanism(s) for inducing DNA fragmentation without the requirement for caspases.
Figures
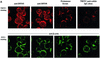

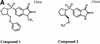
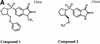
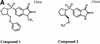
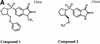
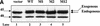
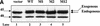
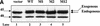
References
-
- Andrade F., Roy,S., Nicholson,D., Thornberry,N., Rosen,A. and Casciola-Rosen,L. (1998) Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity, 8, 451–460. - PubMed
-
- Atkinson E.A., Barry,M., Darmon,A.J., Shostak,I., Turner,P.C., Moyer,R.W. and Bleackley,R.C. (1998) Cytotoxic T lymphocyte-assisted suicide. Caspase 3 activation is primarily the result of the direct action of granzyme B. J. Biol. Chem., 273, 21261–21266. - PubMed
-
- Beidler D.R., Tewari,M., Friesen,P.D., Poirier,G. and Dixit,V.M. (1995) The baculovirus p35 protein inhibits Fas- and tumor necrosis factor-induced apoptosis. J. Biol. Chem., 270, 16526–16528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

