The distal region and receptor tyrosines of the Epo receptor are non-essential for in vivo erythropoiesis
- PMID: 11406592
- PMCID: PMC150206
- DOI: 10.1093/emboj/20.12.3156
The distal region and receptor tyrosines of the Epo receptor are non-essential for in vivo erythropoiesis
Abstract
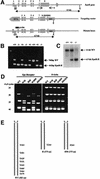
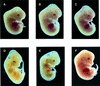
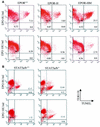
The erythropoietin receptor (EpoR) is required for the proliferation and survival of committed erythroid lineage cells. Previous studies have utilized receptor mutations to show the requirement for the distal half of the cytoplasmic domain of the EpoR and receptor tyrosines for activation of signaling pathways potentially critical to Epo function. To extend these studies to in vivo erythropoiesis, we have created two mutant strains of mice. One strain (H) contains a truncation of the distal half of the cytoplasmic domain, while the second strain (HM) contains the same truncation as well as the mutation of the residual tyrosine (Y(343)) to a phenylalanine. Strikingly, both strains of mice are viable, with only slight alterations in constitutive erythropoiesis or in in vitro assays of red cell lineage function. Challenging H mutant mice with continuous injections of Epo results in an erythrocytosis that is not seen in HM mice. The results demonstrate that neither the distal region nor receptor tyrosines are essential for in vivo EpoR function, but contribute to receptor function in a subtle manner.
Figures
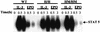



References
-
- Adachi M., Suematsu,S., Kondo,T., Ogasawara,J., Tanaka,T., Yoshida,N. and Nagata,S. (1995) Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genet., 11, 294–300. - PubMed
-
- Arcasoy M.O., Degar,B.A., Harris,K.W. and Forget,B.G. (1997) Familial erythrocytosis associated with a short deletion in the erythropoietin receptor gene. Blood, 89, 4628–4635. - PubMed
-
- Bergelson S., Klingmuller,U., Socolovsky,M., Hsiao,J.G. and Lodish,H.F. (1998) Tyrosine residues within the intracellular domain of the erythropoietin receptor mediate activation of AP-1 transcription factors. J. Biol. Chem., 273, 2396–2401. - PubMed
-
- Chin H., Arai,A., Wakao,H., Kamiyama,R., Miyasaka,N. and Miura,O. (1998) Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway. Blood, 91, 3734–3745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

