Effects of renal function on pharmacokinetics of recombinant human granulocyte colony-stimulating factor in lung cancer patients
- PMID: 11408206
- PMCID: PMC90583
- DOI: 10.1128/AAC.45.7.1947-1951.2001
Effects of renal function on pharmacokinetics of recombinant human granulocyte colony-stimulating factor in lung cancer patients
Abstract
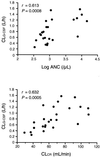
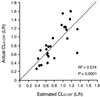
Animal studies suggest that the kidney is involved in the elimination of recombinant human granulocyte colony-stimulating factor (rhG-CSF), which is used for patients with neutropenia during cancer chemotherapy. Since anticancer drugs induce nephrotoxicity, it is important to clarify the role of the kidney in the pharmacokinetics of rhG-CSF in cancer patients. Our study was designed to evaluate the relationship between the pharmacokinetics of rhG-CSF and renal function in lung cancer patients compared to the absolute neutrophil count (ANC). The pharmacokinetic studies were conducted with 25 lung cancer patients. Following chemotherapy using platinum-based compounds, a bolus 5 microg of rhG-CSF/kg of body weight was intravenously injected from the first day of leukopenia or neutropenia. Pharmacokinetic parameters were estimated by fitting the concentration in serum-time data to a two-compartment model according to the population pharmacokinetics and the Bayesian method. Creatinine clearance (CL(CR)) was predicted by the Cockcroft-Gault formula. rhG-CSF clearance (CL(G-CSF)) correlated significantly with the ANC (r = 0.613; P < 0.001) and CL(CR) (r = 0.632; P < 0.001). Multiple linear regression analysis showed that the combination of the ANC and CL(CR) accounted for 57.4% of the variation of CL(G-CSF). In patients with an ANC of <1,000/microl, CL(CR) accounted for 72.9% of the variation of CL(G-CSF) (P < 0.001). Our findings suggest that renal function and neutrophil counts correlate with CL(G-CSF) and that the role of renal function in eliminating rhG-CSF is important in lung cancer patients with neutropenia.
Figures

Similar articles
-
[Efficacy of once-per-cycle administration pegylated recombinant human granulocyte colony-stimulating factor in chemotherapy-induced neutropenia in a phase I trial].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006 Jun;28(3):339-44. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006. PMID: 16900629 Clinical Trial. Chinese.
-
[The effects and pharmacokinetics of rhG-CSF on the treatment of neutropenia in patients with renal failure].Nihon Jinzo Gakkai Shi. 1991 Oct;33(10):973-81. Nihon Jinzo Gakkai Shi. 1991. PMID: 1722829 Clinical Trial. Japanese.
-
The role of polymorphonuclear neutrophils (PMNs) in clearance of granulocyte colony-stimulating factor (G-CSF) in vivo and in vitro.Exp Hematol. 1997 Dec;25(13):1313-25. Exp Hematol. 1997. PMID: 9406990
-
[Clinical study of recombinant human granulocyte colony-stimulating factor (RHG-CSF) on leucopenia induced by chemotherapy with CE regimen on lung cancer patients].Zhonghua Nei Ke Za Zhi. 1994 Nov;33(11):739-42. Zhonghua Nei Ke Za Zhi. 1994. PMID: 7541325 Clinical Trial. Chinese.
-
Close association between clearance of recombinant human granulocyte colony-stimulating factor (G-CSF) and G-CSF receptor on neutrophils in cancer patients.Antimicrob Agents Chemother. 1999 Jan;43(1):21-4. doi: 10.1128/AAC.43.1.21. Antimicrob Agents Chemother. 1999. PMID: 9869559 Free PMC article. Clinical Trial.
Cited by
-
Modelling human granulopoiesis under poly-chemotherapy with G-CSF support.J Math Biol. 2005 Apr;50(4):397-439. doi: 10.1007/s00285-004-0295-1. Epub 2004 Dec 20. J Math Biol. 2005. PMID: 15614553
-
Pharmacokinetics and pharmacodynamics of pegfilgrastim.Clin Pharmacokinet. 2011 May;50(5):295-306. doi: 10.2165/11586040-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21456630 Review.
-
Pegfilgrastim for primary prophylaxis of febrile neutropenia in multiple myeloma.Support Care Cancer. 2021 Nov;29(11):6973-6980. doi: 10.1007/s00520-021-06266-x. Epub 2021 May 14. Support Care Cancer. 2021. PMID: 33990881 Free PMC article. Review.
-
Pegfilgrastim in Supportive Care of Hodgkin Lymphoma.Cancers (Basel). 2022 Aug 23;14(17):4063. doi: 10.3390/cancers14174063. Cancers (Basel). 2022. PMID: 36077600 Free PMC article.
-
Characterization of endogenous G-CSF and the inverse correlation to chemotherapy-induced neutropenia in patients with breast cancer using population modeling.Pharm Res. 2014 Dec;31(12):3390-403. doi: 10.1007/s11095-014-1429-9. Epub 2014 Jun 12. Pharm Res. 2014. PMID: 24919931
References
-
- Akizawa T, Shishido K, Koshikawa S. The effects and pharmacokinetics of rhG-CSF in patients with chronic renal failure. Artif Organs. 1995;19:1251–1257. - PubMed
-
- Aoyagi S, Arasawa K, Matsuyuki A, Kamatchi S, Fukushima M, Ohsawa N. Chemiluminescence sandwich enzyme immunoassay for determination of human granulocyte colony stimulating factor (G-CSF) J Biolumin Chemilumin. 1995;10:345–351. - PubMed
-
- Avalos B R. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996;88:761–777. - PubMed
-
- Beal S L, Sheiner L B. NONMEM users guide. San Francisco: NONMEM Project Group, University of California; 1988.
-
- Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

