Pseudomonas aeruginosa reveals high intrinsic resistance to penem antibiotics: penem resistance mechanisms and their interplay
- PMID: 11408209
- PMCID: PMC90586
- DOI: 10.1128/AAC.45.7.1964-1971.2001
Pseudomonas aeruginosa reveals high intrinsic resistance to penem antibiotics: penem resistance mechanisms and their interplay
Abstract
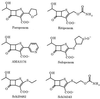
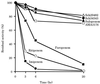
Pseudomonas aeruginosa exhibits high intrinsic resistance to penem antibiotics such as faropenem, ritipenem, AMA3176, sulopenem, Sch29482, and Sch34343. To investigate the mechanisms contributing to penem resistance, we used the laboratory strain PAO1 to construct a series of isogenic mutants with an impaired multidrug efflux system MexAB-OprM and/or impaired chromosomal AmpC beta-lactamase. The outer membrane barrier of PAO1 was partially eliminated by inducing the expression of the plasmid-encoded Escherichia coli major porin OmpF. Susceptibility tests using the mutants and the OmpF expression plasmid showed that MexAB-OprM and the outer membrane barrier, but not AmpC beta-lactamase, are the main mechanisms involved in the high intrinsic penem resistance of PAO1. However, reducing the high intrinsic penem resistance of PAO1 to the same level as that of penem-susceptible gram-negative bacteria such as E. coli required the loss of either both MexAB-OprM and AmpC beta-lactamase or both MexAB-OprM and the outer membrane barrier. Competition experiments for penicillin-binding proteins (PBPs) revealed that the affinity of PBP 1b and PBP 2 for faropenem were about 1.8- and 1.5-fold lower, than the respective affinity for imipenem. Loss of the outer membrane barrier, MexAB, and AmpC beta-lactamase increased the susceptibility of PAO1 to almost all penems tested compared to the susceptibility of the AmpC-deficient PAO1 mutants to imipenem. Thus, it is suggested that the high intrinsic penem resistance of P. aeruginosa is generated from the interplay among the outer membrane barrier, the active efflux system, and AmpC beta-lactamase but not from the lower affinity of PBPs for penems.
Figures

Similar articles
-
Extrusion of penem antibiotics by multicomponent efflux systems MexAB-OprM, MexCD-OprJ, and MexXY-OprM of Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2002 Aug;46(8):2696-9. doi: 10.1128/AAC.46.8.2696-2699.2002. Antimicrob Agents Chemother. 2002. PMID: 12121960 Free PMC article.
-
Multiple genotypic changes in hypersusceptible strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients do not always correlate with the phenotype.J Antimicrob Chemother. 2009 Aug;64(2):294-300. doi: 10.1093/jac/dkp185. Epub 2009 May 25. J Antimicrob Chemother. 2009. PMID: 19468029
-
Hypersusceptibility of the Pseudomonas aeruginosa nfxB mutant to beta-lactams due to reduced expression of the ampC beta-lactamase.Antimicrob Agents Chemother. 2001 Apr;45(4):1284-6. doi: 10.1128/AAC.45.4.1284-1286.2001. Antimicrob Agents Chemother. 2001. PMID: 11257048 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
Roles of porin and beta-lactamase in beta-lactam resistance of Pseudomonas aeruginosa.Rev Infect Dis. 1988 Jul-Aug;10(4):770-5. doi: 10.1093/clinids/10.4.770. Rev Infect Dis. 1988. PMID: 2460909 Review.
Cited by
-
Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria.Microorganisms. 2023 Jun 28;11(7):1690. doi: 10.3390/microorganisms11071690. Microorganisms. 2023. PMID: 37512863 Free PMC article. Review.
-
Distinct Roles of Outer Membrane Porins in Antibiotic Resistance and Membrane Integrity in Escherichia coli.Front Microbiol. 2019 Apr 30;10:953. doi: 10.3389/fmicb.2019.00953. eCollection 2019. Front Microbiol. 2019. PMID: 31114568 Free PMC article.
-
Loss of RNA Chaperone Hfq Unveils a Toxic Pathway in Pseudomonas aeruginosa.J Bacteriol. 2019 Sep 20;201(20):e00232-19. doi: 10.1128/JB.00232-19. Print 2019 Oct 15. J Bacteriol. 2019. PMID: 31358608 Free PMC article.
-
Acquired blaVIM and blaGES Carbapenemase-Encoding Genes in Pseudomonas aeruginosa: A Seven-Year Survey Highlighting an Increasing Epidemiological Threat.Pathogens. 2023 Oct 18;12(10):1256. doi: 10.3390/pathogens12101256. Pathogens. 2023. PMID: 37887772 Free PMC article.
-
Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review.Genes Dis. 2019 Apr 17;6(2):109-119. doi: 10.1016/j.gendis.2019.04.001. eCollection 2019 Jun. Genes Dis. 2019. PMID: 31194018 Free PMC article. Review.
References
-
- Amann E, Ochs B, Abel K J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. - PubMed
-
- Barry A L, Aldrige K E, Allen S D, Fuchs P C, Gerlach E H, Jones R N, Pfaller M A. In vitro activity of FCE22101, imipenem, and ceftazidime against over 6,000 bacterial isolates and MIC quality control limits of FCE22101. Eur J Clin Microbiol Infect Dis. 1988;7:794–798. - PubMed
-
- Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

