Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues
- PMID: 11408227
- PMCID: PMC90604
- DOI: 10.1128/AAC.45.7.2082-2091.2001
Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues
Abstract
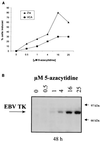
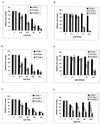
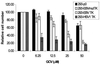
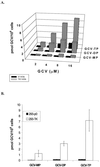
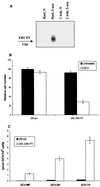
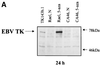
The presence of Epstein-Barr virus (EBV) in the tumor cells of some EBV-associated malignancies may facilitate selective killing of these tumor cells. We show that treatment of an EBV(+) Burkitt's lymphoma cell line with 5-azacytidine led to a dose-dependent induction of EBV lytic antigen expression, including expression of the viral thymidine kinase (TK) and phosphotransferase (PT). Azacytidine treatment for 24 h modestly sensitized the cell line to all nucleosides tested. To better characterize EBV TK with regard to various nucleoside analogues, we expressed EBV TK in stable cell clones. Two EBV TK-expressing clones were moderately sensitive to high doses of acyclovir and penciclovir (PCV) (62.5 to 500 microM) and to lower doses of ganciclovir (GCV) and bromovinyldeoxyuridine (BVdU) (10 to 100 microM) compared to a control clone and were shown to phosphorylate GCV. Similar experiments in a transient overexpression system showed more killing of cells transfected with the EBV TK expression vector than of cells transfected with the control mutant vector (50 microM GCV for 4 days). A putative PT was also studied in the transient transfection system and appeared similar to the TK in phosphorylating GCV and conferring sensitivity to GCV, but not in BVdU- or PCV-mediated cell killing. Induction of EBV kinases in combination with agents such as GCV merits further evaluation as an alternative strategy to gene therapy for selective killing of EBV-infected cells.
Figures


Similar articles
-
The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production.J Virol. 2010 May;84(9):4534-42. doi: 10.1128/JVI.02487-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181711 Free PMC article.
-
Targeted therapy for Epstein-Barr virus-associated gastric carcinoma using low-dose gemcitabine-induced lytic activation.Oncotarget. 2015 Oct 13;6(31):31018-29. doi: 10.18632/oncotarget.5041. Oncotarget. 2015. PMID: 26427042 Free PMC article.
-
The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase.Antimicrob Agents Chemother. 1998 Nov;42(11):2923-31. doi: 10.1128/AAC.42.11.2923. Antimicrob Agents Chemother. 1998. PMID: 9797227 Free PMC article.
-
Experimental treatment of Epstein-Barr virus-associated primary central nervous system lymphoma.Cancer Res. 2003 Mar 1;63(5):965-71. Cancer Res. 2003. PMID: 12615710
-
Induction of the Epstein-Barr virus thymidine kinase gene with concomitant nucleoside antivirals as a therapeutic strategy for Epstein-Barr virus-associated malignancies.Curr Opin Oncol. 2001 Sep;13(5):360-7. doi: 10.1097/00001622-200109000-00008. Curr Opin Oncol. 2001. PMID: 11555713 Review.
Cited by
-
Co-treatment with arsenic trioxide and ganciclovir reduces tumor volume in a murine xenograft model of nasopharyngeal carcinoma.Virol J. 2013 May 16;10:152. doi: 10.1186/1743-422X-10-152. Virol J. 2013. PMID: 23680002 Free PMC article.
-
Use of adenovirus vectors expressing Epstein-Barr virus (EBV) immediate-early protein BZLF1 or BRLF1 to treat EBV-positive tumors.J Virol. 2002 Nov;76(21):10951-9. doi: 10.1128/jvi.76.21.10951-10959.2002. J Virol. 2002. PMID: 12368338 Free PMC article.
-
Development of a novel inducer for EBV lytic therapy.Bioorg Med Chem Lett. 2019 Aug 15;29(16):2259-2264. doi: 10.1016/j.bmcl.2019.06.034. Epub 2019 Jun 22. Bioorg Med Chem Lett. 2019. PMID: 31255485 Free PMC article.
-
Activation of Epstein-Barr Virus' Lytic Cycle in Nasopharyngeal Carcinoma Cells by NEO212, a Conjugate of Perillyl Alcohol and Temozolomide.Cancers (Basel). 2024 Feb 26;16(5):936. doi: 10.3390/cancers16050936. Cancers (Basel). 2024. PMID: 38473298 Free PMC article.
-
Viral response to chemotherapy in endemic burkitt lymphoma.Clin Cancer Res. 2010 Apr 1;16(7):2055-64. doi: 10.1158/1078-0432.CCR-09-2424. Epub 2010 Mar 16. Clin Cancer Res. 2010. PMID: 20233888 Free PMC article. Clinical Trial.
References
-
- Ben-Sasson S A, Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981;28:131–135. - PubMed
-
- Bordignon C, Bonini C, Verzeletti S, Nobili N, Maggioni D, Traversari C, Giavazzi R, Servida P, Zappone E, Benazzi E, et al. Transfer of the HSV-tk gene into donor peripheral blood lymphocytes for in vivo modulation of donor anti-tumor immunity after allogeneic bone marrow transplantation. Hum Gene Ther. 1995;6:813–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

