Pharmacodynamic evaluation of RWJ-270201, a novel neuraminidase inhibitor, in a lethal murine model of influenza predicts efficacy for once-daily dosing
- PMID: 11408232
- PMCID: PMC90609
- DOI: 10.1128/AAC.45.7.2115-2118.2001
Pharmacodynamic evaluation of RWJ-270201, a novel neuraminidase inhibitor, in a lethal murine model of influenza predicts efficacy for once-daily dosing
Abstract
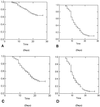
We examined RWJ-270201 in a lethal model of influenza in BALB/c mice. The aim was to delineate the pharmacodynamically linked variable for the drug. Challenge was performed with influenza virus A/Shongdong/09/93 (H3N2). Treatment was administered by gavage. Five doses (1 to 10 mg/kg of body weight) and three schedules (every 24, 12, and 8 h) were evaluated with 10 mice per group. There were 39 placebo-treated mice. Drug exposure was evaluated for infected mice. Exposures were calculated after population modeling of all the plasma concentration-time data simultaneously using the NPEM3 program. Evaluation of dose and schedule with Kaplan-Meier analysis and Cox proportional hazards modeling demonstrated that schedule offered no explanatory power relative to dose alone. Evaluation of peak concentration, trough concentration, and area under the concentration-time curve (AUC) by the same methods revealed that AUC was the dynamically linked variable. Again, schedule offered no further explanatory power when included in the model with AUC. This indicates that AUC is the linked variable and that the anti-influenza effect of RWJ-270201 is independent of schedule. We conclude that once-daily dosing of RWJ-270201 should be evaluated in clinical trials of influenza therapy.
Figures
References
-
- Couch R B. Drug therapy: prevention and treatment of influenza. N Engl J Med. 2000;343:1778–1787. - PubMed
-
- Deguchi Y, Takasugi Y, Tatara K. Efficacy of influenza vaccine in the elderly in welfare nursing homes: reduction in risks in mortality and morbidity during an influenza A (H3N2) epidemic. J Med Microbiol. 2000;49:553–556. - PubMed
-
- Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279:125–129. - PubMed
-
- Schentag J J, Smith I L, Swanson D J, DeAngelis C, Fracasso J E, Vari A, Vance J W. Role for dual individualization with cefmenoxime. Am J Med. 1984;77:43–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources