Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice
- PMID: 11408568
- PMCID: PMC37324
- DOI: 10.1091/mbc.12.6.1557
Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice
Abstract
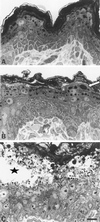
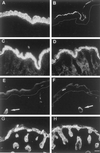
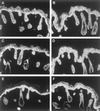
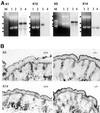
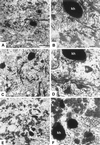
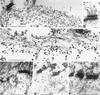
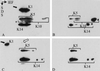
The expression of distinct keratin pairs during epidermal differentiation is assumed to fulfill specific and essential cytoskeletal functions. This is supported by a great variety of genodermatoses exhibiting tissue fragility because of keratin mutations. Here, we show that the loss of K10, the most prominent epidermal protein, allowed the formation of a normal epidermis in neonatal mice without signs of fragility or wound-healing response. However, there were profound changes in the composition of suprabasal keratin filaments. K5/14 persisted suprabasally at elevated protein levels, whereas their mRNAs remained restricted to the basal keratinocytes. This indicated a novel mechanism regulating keratin turnover. Moreover, the amount of K1 was reduced. In the absence of its natural partner we observed the formation of a minor amount of novel K1/14/15 filaments as revealed by immunogold electron microscopy. We suggest that these changes maintained epidermal integrity. Furthermore, suprabasal keratinocytes contained larger keratohyalin granules similar to our previous K10T mice. A comparison of profilaggrin processing in K10T and K10(-/-) mice revealed an accumulation of filaggrin precursors in the former but not in the latter, suggesting a requirement of intact keratin filaments for the processing. The mild phenotype of K10(-/-) mice suggests that there is a considerable redundancy in the keratin gene family.
Figures



Similar articles
-
Mouse differentiation-specific keratins 1 and 10 require a preexisting keratin scaffold to form a filament network.J Cell Biol. 1993 Mar;120(5):1251-61. doi: 10.1083/jcb.120.5.1251. J Cell Biol. 1993. PMID: 7679677 Free PMC article.
-
Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex.Mol Biol Cell. 2001 Jun;12(6):1775-89. doi: 10.1091/mbc.12.6.1775. Mol Biol Cell. 2001. PMID: 11408584 Free PMC article.
-
Deletion of K1/K10 does not impair epidermal stratification but affects desmosomal structure and nuclear integrity.J Cell Sci. 2012 Apr 1;125(Pt 7):1750-8. doi: 10.1242/jcs.097139. Epub 2012 Feb 28. J Cell Sci. 2012. PMID: 22375063
-
Lessons from disorders of epidermal differentiation-associated keratins.Histol Histopathol. 2002 Jan;17(1):331-8. doi: 10.14670/HH-17.331. Histol Histopathol. 2002. PMID: 11813882 Review.
-
Keratohyalin, trichohyalin and keratohyalin-trichohyalin hybrid granules: an overview.J Dermatol. 1992 Nov;19(11):749-55. doi: 10.1111/j.1346-8138.1992.tb03774.x. J Dermatol. 1992. PMID: 1284067 Review.
Cited by
-
The expanding significance of keratin intermediate filaments in normal and diseased epithelia.Curr Opin Cell Biol. 2013 Feb;25(1):47-56. doi: 10.1016/j.ceb.2012.10.018. Epub 2012 Dec 25. Curr Opin Cell Biol. 2013. PMID: 23270662 Free PMC article. Review.
-
Epidermolysis bullosa simplex-type mutations alter the dynamics of the keratin cytoskeleton and reveal a contribution of actin to the transport of keratin subunits.Mol Biol Cell. 2004 Mar;15(3):990-1002. doi: 10.1091/mbc.e03-09-0687. Epub 2003 Dec 10. Mol Biol Cell. 2004. PMID: 14668478 Free PMC article.
-
A Small Indel Mutant Mouse Model of Epidermolytic Palmoplantar Keratoderma and Its Application to Mutant-specific shRNA Therapy.Mol Ther Nucleic Acids. 2016 Mar 22;5(3):e299. doi: 10.1038/mtna.2016.17. Mol Ther Nucleic Acids. 2016. PMID: 27003758 Free PMC article.
-
Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin.PLoS Pathog. 2012 Dec;8(12):e1003092. doi: 10.1371/journal.ppat.1003092. Epub 2012 Dec 27. PLoS Pathog. 2012. PMID: 23300445 Free PMC article.
-
Arginine- but not alanine-rich carboxy-termini trigger nuclear translocation of mutant keratin 10 in ichthyosis with confetti.J Cell Mol Med. 2019 Dec;23(12):8442-8452. doi: 10.1111/jcmm.14727. Epub 2019 Oct 22. J Cell Mol Med. 2019. PMID: 31638346 Free PMC article.
References
-
- Baribault H, Penner J, Iozzo RV, Wilson HM. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. - PubMed
-
- Batta K, Rugg EL, Wilson NJ, West N, Goodyear H, Lane EB, Gratian M, Dopping-Hepenstal P, Moss C, Eady RA. A. keratin 14 “knockout” mutation in recessive epidermolysis bullosa simplex resulting in less severe disease. Br J Dermatol. 2000;143:621–627. - PubMed
-
- Bickenbach JR, Longley MA, Bundman DS, Dominey AM, Bowden PE, Rothnagel JA, Roop DR. A transgenic mouse model that recapitulates the clinical features of both neonatal and adult forms of the skin disease epidermolytic hyperkeratosis. Differentiation. 1996;61:129–139. - PubMed
-
- Bussow H. Schwann cell myelin ensheathing CNS axons in the nerve fiber layer of the cat retina. J Neurocytol. 1978;7:207–214. - PubMed
-
- Candi E, Tarcsa E, DiGiovanna JJ, Compton JG, Elias PM, Marekov LN, Steinert PM. A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proc Natl Acad Sci USA. 1998;95:2067–2072. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

