Characterization of a novel human SMC heterodimer homologous to the Schizosaccharomyces pombe Rad18/Spr18 complex
- PMID: 11408570
- PMCID: PMC37326
- DOI: 10.1091/mbc.12.6.1583
Characterization of a novel human SMC heterodimer homologous to the Schizosaccharomyces pombe Rad18/Spr18 complex
Abstract
The structural maintenance of chromosomes (SMC) protein encoded by the fission yeast rad18 gene is involved in several DNA repair processes and has an essential function in DNA replication and mitotic control. It has a heterodimeric partner SMC protein, Spr18, with which it forms the core of a multiprotein complex. We have now isolated the human orthologues of rad18 and spr18 and designated them hSMC6 and hSMC5. Both proteins are about 1100 amino acids in length and are 27-28% identical to their fission yeast orthologues, with much greater identity within their N- and C-terminal globular domains. The hSMC6 and hSMC5 proteins interact to form a tight complex analogous to the yeast Rad18/Spr18 heterodimer. In proliferating human cells the proteins are bound to both chromatin and the nucleoskeleton. In addition, we have detected a phosphorylated form of hSMC6 that localizes to interchromatin granule clusters. Both the total level of hSMC6 and its phosphorylated form remain constant through the cell cycle. Both hSMC5 and hSMC6 proteins are expressed at extremely high levels in the testis and associate with the sex chromosomes in the late stages of meiotic prophase, suggesting a possible role for these proteins in meiosis.
Figures





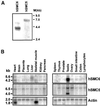
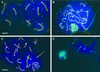
Similar articles
-
A novel SMC protein complex in Schizosaccharomyces pombe contains the Rad18 DNA repair protein.EMBO J. 2000 Apr 3;19(7):1691-702. doi: 10.1093/emboj/19.7.1691. EMBO J. 2000. PMID: 10747036 Free PMC article.
-
Composition and architecture of the Schizosaccharomyces pombe Rad18 (Smc5-6) complex.Mol Cell Biol. 2005 Jan;25(1):172-84. doi: 10.1128/MCB.25.1.172-184.2005. Mol Cell Biol. 2005. PMID: 15601840 Free PMC article.
-
Human and mouse homologs of Schizosaccharomyces pombe rad1(+) and Saccharomyces cerevisiae RAD17: linkage to checkpoint control and mammalian meiosis.Genes Dev. 1998 Aug 15;12(16):2560-73. doi: 10.1101/gad.12.16.2560. Genes Dev. 1998. PMID: 9716408 Free PMC article.
-
Smc5/6, an atypical SMC complex with two RING-type subunits.Biochem Soc Trans. 2020 Oct 30;48(5):2159-2171. doi: 10.1042/BST20200389. Biochem Soc Trans. 2020. PMID: 32964921 Review.
-
FHA-RING ubiquitin ligases in cell division cycle control.Cell Mol Life Sci. 2008 Nov;65(21):3458-66. doi: 10.1007/s00018-008-8220-1. Cell Mol Life Sci. 2008. PMID: 18597043 Free PMC article. Review.
Cited by
-
Coordination of DNA damage responses via the Smc5/Smc6 complex.Mol Cell Biol. 2004 Jan;24(2):662-74. doi: 10.1128/MCB.24.2.662-674.2004. Mol Cell Biol. 2004. PMID: 14701739 Free PMC article.
-
Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor.Nature. 2016 Mar 17;531(7594):386-9. doi: 10.1038/nature17170. Nature. 2016. PMID: 26983541
-
NSMCE2 suppresses cancer and aging in mice independently of its SUMO ligase activity.EMBO J. 2015 Nov 3;34(21):2604-19. doi: 10.15252/embj.201591829. Epub 2015 Oct 6. EMBO J. 2015. PMID: 26443207 Free PMC article.
-
SMC proteins and chromosome mechanics: from bacteria to humans.Philos Trans R Soc Lond B Biol Sci. 2005 Mar 29;360(1455):507-14. doi: 10.1098/rstb.2004.1606. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 15897176 Free PMC article. Review.
-
Comparison study on k-word statistical measures for protein: from sequence to 'sequence space'.BMC Bioinformatics. 2008 Sep 23;9:394. doi: 10.1186/1471-2105-9-394. BMC Bioinformatics. 2008. PMID: 18811946 Free PMC article.
References
-
- Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR, III, Hays L, Morgan WF, Petrini JHJ. The hMre11/hRad50 protein complex and Nijmegen breakage-syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. - PubMed
-
- Cobbe N, Heck MM. Review: SMCs in the world of chromosome biology: from prokaryotes to higher eukaryotes. J Struct Biol. 2000;129:123–143. - PubMed
-
- Deloukas P, Schuler GD, Gyapay G, Beasley EM, Soderlund C, Rodriguez-Tome P, Hui L, Matise TC, McKusick KB, Beckmann JS, Bentolila S, Bihoreau M, Birren BB, Browne J, Butler A, Castle AB, Chiannilkulchai N, Clee C, Day PJ, Dehejia A, Dibling T, Drouot N, Duprat S, Fizames C, Bentley DR, et al. A physical map of 30,000 human genes. Science. 1998;282:744–746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

