alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin
- PMID: 11408571
- PMCID: PMC37327
- DOI: 10.1091/mbc.12.6.1595
alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin
Abstract
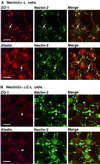
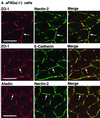
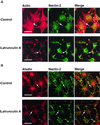
ZO-1 is an actin filament (F-actin)-binding protein that localizes to tight junctions and connects claudin to the actin cytoskeleton in epithelial cells. In nonepithelial cells that have no tight junctions, ZO-1 localizes to adherens junctions (AJs) and may connect cadherin to the actin cytoskeleton indirectly through beta- and alpha-catenins as one of many F-actin-binding proteins. Nectin is an immunoglobulin-like adhesion molecule that localizes to AJs and is associated with the actin cytoskeleton through afadin, an F-actin-binding protein. Ponsin is an afadin- and vinculin-binding protein that also localizes to AJs. The nectin-afadin complex has a potency to recruit the E-cadherin-beta-catenin complex through alpha-catenin in a manner independent of ponsin. By the use of cadherin-deficient L cell lines stably expressing various components of the cadherin-catenin and nectin-afadin systems, and alpha-catenin-deficient F9 cell lines, we examined here whether nectin recruits ZO-1 to nectin-based cell-cell adhesion sites. Nectin showed a potency to recruit not only alpha-catenin but also ZO-1 to nectin-based cell-cell adhesion sites. This recruitment of ZO-1 was dependent on afadin but independent of alpha-catenin and ponsin. These results indicate that ZO-1 localizes to cadherin-based AJs through interactions not only with alpha-catenin but also with the nectin-afadin system.
Figures








Similar articles
-
Involvement of LMO7 in the association of two cell-cell adhesion molecules, nectin and E-cadherin, through afadin and alpha-actinin in epithelial cells.J Biol Chem. 2004 Jul 23;279(30):31365-73. doi: 10.1074/jbc.M401957200. Epub 2004 May 12. J Biol Chem. 2004. PMID: 15140894
-
Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells.J Biol Chem. 2006 Feb 24;281(8):5288-99. doi: 10.1074/jbc.M510070200. Epub 2005 Dec 18. J Biol Chem. 2006. PMID: 16361708
-
Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells.Genes Cells. 1999 Oct;4(10):573-81. doi: 10.1046/j.1365-2443.1999.00283.x. Genes Cells. 1999. PMID: 10583506
-
Nectin and afadin: novel organizers of intercellular junctions.J Cell Sci. 2003 Jan 1;116(Pt 1):17-27. doi: 10.1242/jcs.00167. J Cell Sci. 2003. PMID: 12456712 Review.
-
The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin.Annu Rev Cell Dev Biol. 2008;24:309-42. doi: 10.1146/annurev.cellbio.24.110707.175339. Annu Rev Cell Dev Biol. 2008. PMID: 18593353 Review.
Cited by
-
Junctional adhesion molecule-A: functional diversity through molecular promiscuity.Cell Mol Life Sci. 2018 Apr;75(8):1393-1409. doi: 10.1007/s00018-017-2729-0. Epub 2017 Dec 14. Cell Mol Life Sci. 2018. PMID: 29238845 Free PMC article. Review.
-
Quantitative phosphotyrosine proteomics of EphB2 signaling by stable isotope labeling with amino acids in cell culture (SILAC).J Proteome Res. 2006 Mar;5(3):581-8. doi: 10.1021/pr050362b. J Proteome Res. 2006. PMID: 16512673 Free PMC article.
-
Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the "Yin" and "Yang" effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2.Int Rev Cell Mol Biol. 2013;301:291-358. doi: 10.1016/B978-0-12-407704-1.00006-3. Int Rev Cell Mol Biol. 2013. PMID: 23317821 Free PMC article. Review.
-
Afadin Downregulation by Helicobacter pylori Induces Epithelial to Mesenchymal Transition in Gastric Cells.Front Microbiol. 2018 Nov 9;9:2712. doi: 10.3389/fmicb.2018.02712. eCollection 2018. Front Microbiol. 2018. PMID: 30473688 Free PMC article.
-
Proximity proteomics identifies PAK4 as a component of Afadin-Nectin junctions.Nat Commun. 2021 Sep 7;12(1):5315. doi: 10.1038/s41467-021-25011-w. Nat Commun. 2021. PMID: 34493720 Free PMC article.
References
-
- Ando-Akatsuka Y, Yonemura S, Itoh M, Furuse M, Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol. 1996;179:115–125. - PubMed
-
- Aoki J, Koike S, Ise I, Sato-Yoshida Y, Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J Biol Chem. 1994;269:8431–8438. - PubMed
-
- Asakura T, Nakanishi H, Sakisaka T, Takahashi K, Mandai K, Nishimura M, Sasaki T, Takai Y. Similar and differential behavior between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells. 1999;4:573–581. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

