A novel quality control compartment derived from the endoplasmic reticulum
- PMID: 11408579
- PMCID: PMC37335
- DOI: 10.1091/mbc.12.6.1711
A novel quality control compartment derived from the endoplasmic reticulum
Abstract
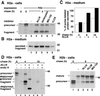
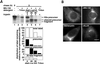
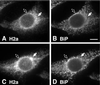
Degradation of proteins that, because of improper or suboptimal processing, are retained in the endoplasmic reticulum (ER) involves retrotranslocation to reach the cytosolic ubiquitin-proteasome machinery. We found that substrates of this pathway, the precursor of human asialoglycoprotein receptor H2a and free heavy chains of murine class I major histocompatibility complex (MHC), accumulate in a novel preGolgi compartment that is adjacent to but not overlapping with the centrosome, the Golgi complex, and the ER-to-Golgi intermediate compartment (ERGIC). On its way to degradation, H2a associated increasingly after synthesis with the ER translocon Sec61. Nevertheless, it remained in the secretory pathway upon proteasomal inhibition, suggesting that its retrotranslocation must be tightly coupled to the degradation process. In the presence of proteasomal inhibitors, the ER chaperones calreticulin and calnexin, but not BiP, PDI, or glycoprotein glucosyltransferase, concentrate in the subcellular region of the novel compartment. The "quality control" compartment is possibly a subcompartment of the ER. It depends on microtubules but is insensitive to brefeldin A. We discuss the possibility that it is also the site for concentration and retrotranslocation of proteins that, like the mutant cystic fibrosis transmembrane conductance regulator, are transported to the cytosol, where they form large aggregates, the "aggresomes."
Figures






Similar articles
-
Ubiquitination is required for the retro-translocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome.J Biol Chem. 1998 Apr 17;273(16):9734-43. doi: 10.1074/jbc.273.16.9734. J Biol Chem. 1998. PMID: 9545309
-
Separate roles and different routing of calnexin and ERp57 in endoplasmic reticulum quality control revealed by interactions with asialoglycoprotein receptor chains.Mol Biol Cell. 2004 May;15(5):2133-42. doi: 10.1091/mbc.e03-12-0899. Epub 2004 Feb 20. Mol Biol Cell. 2004. PMID: 14978212 Free PMC article.
-
CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway.J Virol. 1998 Mar;72(3):2280-8. doi: 10.1128/JVI.72.3.2280-2288.1998. J Virol. 1998. PMID: 9499087 Free PMC article.
-
Ubiquitin and the control of protein fate in the secretory and endocytic pathways.Annu Rev Cell Dev Biol. 1998;14:19-57. doi: 10.1146/annurev.cellbio.14.1.19. Annu Rev Cell Dev Biol. 1998. PMID: 9891777 Free PMC article. Review.
-
ER quality control: towards an understanding at the molecular level.Curr Opin Cell Biol. 2001 Aug;13(4):431-7. doi: 10.1016/s0955-0674(00)00233-7. Curr Opin Cell Biol. 2001. PMID: 11454449 Review.
Cited by
-
EDEM1 reveals a quality control vesicular transport pathway out of the endoplasmic reticulum not involving the COPII exit sites.Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4407-12. doi: 10.1073/pnas.0700154104. Epub 2007 Mar 6. Proc Natl Acad Sci U S A. 2007. PMID: 17360537 Free PMC article.
-
ERAD and how viruses exploit it.Front Microbiol. 2014 Jul 3;5:330. doi: 10.3389/fmicb.2014.00330. eCollection 2014. Front Microbiol. 2014. PMID: 25071743 Free PMC article. Review.
-
The Emerging Roles of Early Protein Folding Events in the Secretory Pathway in the Development of Neurodegenerative Maladies.Front Neurosci. 2017 Feb 7;11:48. doi: 10.3389/fnins.2017.00048. eCollection 2017. Front Neurosci. 2017. PMID: 28223916 Free PMC article. Review.
-
Pet, a non-AB toxin, is transported and translocated into epithelial cells by a retrograde trafficking pathway.Infect Immun. 2007 May;75(5):2101-9. doi: 10.1128/IAI.01515-06. Epub 2007 Feb 12. Infect Immun. 2007. PMID: 17296748 Free PMC article.
-
Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications.FEBS J. 2019 Jan;286(2):241-278. doi: 10.1111/febs.14608. Epub 2018 Aug 4. FEBS J. 2019. PMID: 30027602 Free PMC article. Review.
References
-
- Ayalon-Soffer M, Kamhi-Nesher S, Lederkremer GZ. Folding and self-assembly do not prevent ER retention and proteasomal degradation of asialoglycoprotein receptor H2a. FEBS Lett. 1999a;460:112–116. - PubMed
-
- Ayalon-Soffer M, Shenkman M, Lederkremer GZ. Differential role of mannose and glucose trimming in the ER degradation of asialoglycoprotein receptor subunits. J Cell Sci. 1999b;112:3309–3318. - PubMed
-
- Bebok Z, Mazzochi C, King SA, Hong JS, Sorscher EJ. The mechanism underlying cystic fibrosis transmembrane conductance regulator transport from the endoplasmic reticulum to the proteasome includes Sec61β and a cytosolic, deglycosylated intermediary. J Biol Chem. 1998;273:29873–29878. - PubMed
-
- Biederer T, Volkwein C, Sommer T. Role of Cue1p in ubiquitination and degradation at the ER surface. Science. 1997;278:1806–1809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

