Distribution and transport of cholesterol in Caenorhabditis elegans
- PMID: 11408580
- PMCID: PMC37336
- DOI: 10.1091/mbc.12.6.1725
Distribution and transport of cholesterol in Caenorhabditis elegans
Abstract
Cholesterol transport is an essential process in all multicellular organisms. In this study we applied two recently developed approaches to investigate the distribution and molecular mechanisms of cholesterol transport in Caenorhabditis elegans. The distribution of cholesterol in living worms was studied by imaging its fluorescent analog, dehydroergosterol, which we applied to the animals by feeding. Dehydroergosterol accumulates primarily in the pharynx, nerve ring, excretory gland cell, and gut of L1-L3 larvae. Later, the bulk of dehydroergosterol accumulates in oocytes and spermatozoa. Males display exceptionally strong labeling of spermatids, which suggests a possible role for cholesterol in sperm development. In a complementary approach, we used a photoactivatable cholesterol analog to identify cholesterol-binding proteins in C. elegans. Three major and several minor proteins were found specifically cross-linked to photocholesterol after UV irradiation. The major proteins were identified as vitellogenins. rme-2 mutants, which lack the vitellogenin receptor, fail to accumulate dehydroergosterol in oocytes and embryos and instead accumulate dehydroergosterol in the body cavity along with vitellogenin. Thus, uptake of cholesterol by C. elegans oocytes occurs via an endocytotic pathway involving yolk proteins. The pathway is a likely evolutionary ancestor of mammalian cholesterol transport.
Figures




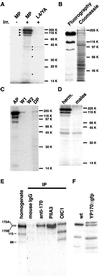

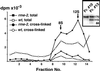
References
-
- Austin CR. The 'capacitation' of the mammalian sperm. Nature. 1952;170:326. - PubMed
-
- Bargmann CI, Mori I . a.C.o.C.e. Researchers, editors. Chemotaxis and thermotaxis. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 717–737.
-
- Bottjer KP, Weinstein PP, Thompson MJ. Effects of an azasteroid on growth, development and reproduction of the free-living nematodes Caenorhabditis briggsae and Panagrellus redivivus. Comp Biochem Physiol. 1985;82B:99–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

