Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos
- PMID: 11408582
- PMCID: PMC37338
- DOI: 10.1091/mbc.12.6.1751
Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos
Abstract
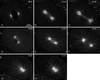
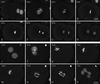
gamma-Tubulin is a ubiquitous and highly conserved component of centrosomes in eukaryotic cells. Genetic and biochemical studies have demonstrated that gamma-tubulin functions as part of a complex to nucleate microtubule polymerization from centrosomes. We show that, as in other organisms, Caenorhabditis elegans gamma-tubulin is concentrated in centrosomes. To study centrosome dynamics in embryos, we generated transgenic worms that express GFP::gamma-tubulin or GFP::beta-tubulin in the maternal germ line and early embryos. Multiphoton microscopy of embryos produced by these worms revealed the time course of daughter centrosome appearance and growth and the differential behavior of centrosomes destined for germ line and somatic blastomeres. To study the role of gamma-tubulin in nucleation and organization of spindle microtubules, we used RNA interference (RNAi) to deplete C. elegans embryos of gamma-tubulin. gamma-Tubulin (RNAi) embryos failed in chromosome segregation, but surprisingly, they contained extensive microtubule arrays. Moderately affected embryos contained bipolar spindles with dense and long astral microtubule arrays but with poorly organized kinetochore and interpolar microtubules. Severely affected embryos contained collapsed spindles with numerous long astral microtubules. Our results suggest that gamma-tubulin is not absolutely required for microtubule nucleation in C. elegans but is required for the normal organization and function of kinetochore and interpolar microtubules.
Figures




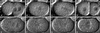

References
-
- Albertson DG. Formation of the first cleavage spindle in nematode embryos. Dev Biol. 1984;101:61–72. - PubMed
-
- Albertson DG, Thomson JN. Segregation of holocentric chromosomes at meiosis in the nematode Caenorhabditis elegans. Chromosome Res. 1993;1:15–26. - PubMed
-
- Bobinnec Y, Fukuda M, Nishida E. Identification and characterization of Caenorhabditis elegansgamma-tubulin in dividing cells and differentiated tissues. J Cell Sci. 2000;113:3747–3759. - PubMed
-
- Consortium, T.C.e.S. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

