Myosin vb is associated with plasma membrane recycling systems
- PMID: 11408590
- PMCID: PMC37346
- DOI: 10.1091/mbc.12.6.1843
Myosin vb is associated with plasma membrane recycling systems
Abstract
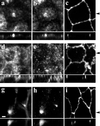
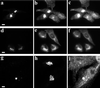
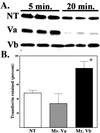
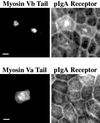
Myosin Va is associated with discrete vesicle populations in a number of cell types, but little is known of the function of myosin Vb. Yeast two-hybrid screening of a rabbit parietal cell cDNA library with dominant active Rab11a (Rab11aS20V) identified myosin Vb as an interacting protein for Rab11a, a marker for plasma membrane recycling systems. The isolated clone, corresponding to the carboxyl terminal 60 kDa of the myosin Vb tail, interacted with all members of the Rab11 family (Rab11a, Rab11b, and Rab25). GFP-myosin Vb and endogenous myosin Vb immunoreactivity codistributed with Rab11a in HeLa and Madin-Darby canine kidney (MDCK) cells. As with Rab11a in MDCK cells, the myosin Vb immunoreactivity was dispersed with nocodazole treatment and relocated to the apical corners of cells with taxol treatment. A green fluorescent protein (GFP)-myosin Vb tail chimera overexpressed in HeLa cells retarded transferrin recycling and caused accumulation of transferrin and the transferrin receptor in pericentrosomal vesicles. Expression of the myosin Vb tail chimera in polarized MDCK cells stably expressing the polymeric IgA receptor caused accumulation of basolaterally endocytosed polymeric IgA and the polymeric IgA receptor in the pericentrosomal region. The myosin Vb tail had no effects on transferrin trafficking in polarized MDCK cells. The GFP-myosin Va tail did not colocalize with Rab11a and had no effects on recycling system vesicle distribution in either HeLa or MDCK cells. The results indicate myosin Vb is associated with the plasma membrane recycling system in nonpolarized cells and the apical recycling system in polarized cells. The dominant negative effects of the myosin Vb tail chimera indicate that this unconventional myosin is required for transit out of plasma membrane recycling systems.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

