casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish
- PMID: 11410530
- PMCID: PMC312713
- DOI: 10.1101/gad.892301
casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish
Abstract
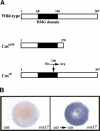
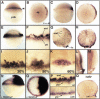
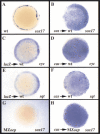
Early endoderm formation in zebrafish requires at least three loci that function downstream of Nodal signaling but upstream of the early endodermal marker sox17: bonnie and clyde (bon), faust (fau), and casanova (cas). cas mutants show the most severe phenotype as they do not form any gut tissue and lack all sox17 expression. Activation of the Nodal signaling pathway or overexpression of Bon or Fau/Gata5 fails to restore any sox17 expression in cas mutants, demonstrating that cas plays a central role in endoderm formation. Here we show that cas encodes a novel member of the Sox family of transcription factors. Initial cas expression appears in the dorsal yolk syncytial layer (YSL) in the early blastula, and is independent of Nodal signaling. In contrast, endodermal expression of cas, which begins in the late blastula, is regulated by Nodal signaling. Cas is a potent inducer of sox17 expression in wild-type embryos as well as in bon and fau/gata5 mutants. Cas is also a potent inducer of sox17 expression in MZoep mutants, which cannot respond to Nodal signaling. In addition, ectopic expression of cas in presumptive mesodermal cells leads to their transfating into endoderm. Altogether, these data indicate that Cas is the principal transcriptional effector of Nodal signaling during zebrafish endoderm formation.
Figures






References
-
- Alexander J, Stainier DYR. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–1157. - PubMed
-
- Alexander J, Stainier DYR, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev Genet. 1998;22:288–299. - PubMed
-
- Alexander J, Rothenberg M, Henry GL, Stainier DYR. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. - PubMed
-
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–255. - PubMed
-
- Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJM, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
