The lac operator-repressor system is functional in the mouse
- PMID: 11410531
- PMCID: PMC312721
- DOI: 10.1101/gad.892001
The lac operator-repressor system is functional in the mouse
Abstract
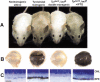
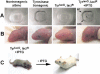
We report the successful transfer of a fully functional lac operator-repressor gene regulatory system to the mouse. The key component is a lac repressor transgene that resembles a typical mammalian gene both in codon usage and structure and expresses functional levels of repressor protein in the animal. We used the repressor to regulate the expression of a mammalian reporter gene consisting of the tyrosinase promoter embedded with three short lac operator sequences and the tyrosinase coding sequence. Pigmentation of the mouse was controlled by the interaction of the lac repressor with the regulatable Tyrosinase transgene in a manner that was fully reversible by the lactose analog IPTG. Direct control of mammalian promoters by the lac repressor provides tight, reversible regulation, predictable levels of de-repressed expression, and the promise of reversible control of the endogenous genome.
Figures



Comment in
-
Changing colors in mice: an inducible system that delivers.Genes Dev. 2001 Jun 15;15(12):1461-7. doi: 10.1101/gad.909301. Genes Dev. 2001. PMID: 11410526 Review. No abstract available.
References
-
- Brown M, Figge J, Hansen U, Wright C, Jeang KT, Khoury G, Livingston DM, Roberts TM. lac repressor can regulate expression from a hybrid SV40 early promoter containing a lac operator in animal cells. Cell. 1987;49:603–612. - PubMed
-
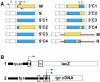
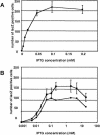
- Cho S, Scharpf S, Franko M, Vermeulen CW. Effect of iso-propyl-thio-beta-D-galactoside concentration on the level of lac-operon induction in steady state Escherichia coli. Biochem Biophys Res Commun. 1985;128:1268–1273. - PubMed
-
- Drager UC. Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc R Soc Lond B Biol Sci. 1985;224:57–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
