Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene
- PMID: 11410537
- PMCID: PMC312715
- DOI: 10.1101/gad.887301
Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene
Abstract
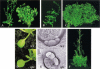
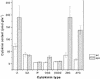
The aerial architecture of flowering plants is determined to a large extent by shoot growth and shoot branching arising from the initiation and growth of axillary meristems. We have identified an Arabidopsis mutant, supershoot (sps), which is characterized by a massive overproliferation of shoots, such that a single plant can generate 500 or more inflorescences. Analysis of the mutant plants shows that the primary defect is because of an increase in the number of meristems formed in leaf axils, together with release of bud arrest, resulting in reiterative branch formation from rosette and cauline leaves. The SPS gene is shown here to encode a cytochrome P450, and together with a 3- to 9-fold increase in levels of Z-type cytokinins in sps mutant plants, indicate a role for SPS in modulating hormone levels. The expression pattern of SPS, with strong expression at the leaf axils, correlates well with the phenotypic defects. Our results indicate that control of shoot branching in Arabidopsis may be accomplished in part by suppression of axillary meristem initiation and growth through the localized attenuation of cytokinin levels at sites of bud initiation.
Figures




References
-
- Bak S, Nielsen HL, Halkier BA. The presence of CYP79 homologues in glucosinolate-producing plants shows evolutinary conservation of the enzymes in the conversion of amino acid to aldoxime in the biosynthesis of cyanogenic glucosides and glucosinolates. Plant Mol Biol. 1998;38:725–734. - PubMed
-
- Balcells L, Sundberg E, Coupland G. A heat-shock promoter fusion to the Ac transposase gene drives inducible transposition of a Ds element during Arabidopsis embryo development. Plant J. 1994;5:755–764.
-
- Bangerth F. Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment and relationship to apical dominance. Planta. 1994;194:439–442.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
