Siglecs in the immune system
- PMID: 11412300
- PMCID: PMC1783234
- DOI: 10.1046/j.0019-2805.2001.01241.x
Siglecs in the immune system
Figures
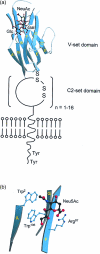
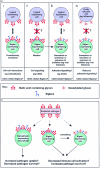
References
-
- Crocker PR, Clark EA, Filbin M, et al. Siglecs: a family of sialic-acid binding lectins [letter] Glycobiology. 1998;8:v. - PubMed
-
- Powell LD, Varki A. I-type lectins. J Biol Chem. 1995;270:14243–6. - PubMed
-
- May AP, Robinson RC, Vinson M, Crocker PR, Jones EY. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3′ sialyllactose at 1.85 Å resolution. Mol Cell. 1998;1:719–28. - PubMed
-
- Stamenkovic I, Seed B. The B-cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990;345:74–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

