Detection of anaphylatoxin receptors on CD83+ dendritic cells derived from human skin
- PMID: 11412308
- PMCID: PMC1783227
- DOI: 10.1046/j.1365-2567.2001.01197.x
Detection of anaphylatoxin receptors on CD83+ dendritic cells derived from human skin
Abstract
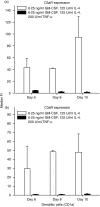
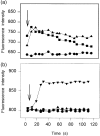
Dendritic cells (DC) are recruited to sites of inflammation for the initiation of immune responses. As the anaphylatoxins C5a and C3a are important mediators of inflammation, we investigated the expression of their receptors (C3aR and C5aR) on human DC. DC were isolated from human skin or generated from purified blood monocytes and were identified by their expression of CD1a or CD83. Freshly isolated or cultured dermal CD1a+ and CD83+ DC bound anti-C5aR and anti-C3aR monoclonal antibodies (mAbs), as detected by flow cytometry. C5a induced calcium fluxes in dermal CD1a+ and CD83+ DC, which could be inhibited by C17/5, an anti-C5a mAb. C3a did not induce calcium fluxes in these cells. Anaphylatoxin receptor expression was down-regulated on dermal DC by adding tumour necrosis factor-alpha (TNF-alpha) to the culture medium. On CD1a+ CD83- cells generated from isolated blood monocytes by culture with 6.25 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF) and 125 U/ml of interleukin-4 (IL-4), expression of both C5aR and C3aR was observed. In these cells, both C5a and C3a induced calcium fluxes. After addition of TNF-alpha to the culture medium, the majority of the CD1a+ cells expressed CD83+. These cells - expressing a phenotype of 'mature DC' - down-regulated the expression of the anaphylatoxin receptors and lost their reactivity to the respective ligands. Our results demonstrate the expression of the anaphylatoxin receptors C5aR and C3aR on human skin-derived DC and blood-derived cells expressing the DC-associated membrane molecule, CD1a. Furthermore, the expression of anaphylatoxin receptors on CD83+ dermal DC is indicative of an intermediate stage of maturation of these cells, which was not observed on in vitro-differentiated CD83+ cells.
Figures





References
-
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75–8. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. - PubMed
-
- Caux C, Liu YJ, Banchereau J. Recent advances in the study of dendritic cells and follicular dendritic cells. Immunol Today. 1995;16:2–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

