Unorthodox routes to prostanoid formation: new twists in cyclooxygenase-initiated pathways
- PMID: 11413151
- PMCID: PMC200204
- DOI: 10.1172/JCI13375
Unorthodox routes to prostanoid formation: new twists in cyclooxygenase-initiated pathways
Figures

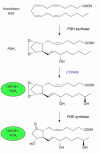

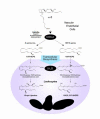
References
-
- Bylund J, Hidestrand M, Ingelman-Sundberg M, Oliw EH. Identification of CYP4F8 in human seminal vesicles as a prominent 19- hydroxylase of prostaglandin endoperoxides. J Biol Chem. 2000;275:21844–21849. - PubMed
-
- Bergström, S. 1982. The prostaglandins: from the laboratory to the clinic. In Les prix nobel: Nobel prizes, presentations, biographies and lectures. Edited by The Nobel Foundation. Almqvist & Wiksell. Stockholm, Sweden. 129–148.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

