Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation
- PMID: 11413191
- PMCID: PMC2193303
- DOI: 10.1084/jem.193.12.1361
Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation
Abstract
Promyelocytic leukemia (PML) is the organizer of nuclear matrix domains, PML nuclear bodies (NBs), with a proposed role in apoptosis control. In acute promyelocytic leukemia, PML/retinoic acid receptor (RAR) alpha expression disrupts NBs, but therapies such as retinoic acid or arsenic trioxide (As2O3) restore them. PML is conjugated by the ubiquitin-related peptide SUMO-1, a process enhanced by As2O3 and proposed to target PML to the nuclear matrix. We demonstrate that As2O3 triggers the proteasome-dependent degradation of PML and PML/RARalpha and that this process requires a specific sumolation site in PML, K160. PML sumolation is dispensable for its As2O3-induced matrix targeting and formation of primary nuclear aggregates, but is required for the formation of secondary shell-like NBs. Interestingly, only these mature NBs harbor 11S proteasome components, which are further recruited upon As2O3 exposure. Proteasome recruitment by sumolated PML only likely accounts for the failure of PML-K160R to be degraded. Therefore, studying the basis of As2O3-induced PML/RARalpha degradation we show that PML sumolation directly or indirectly promotes its catabolism, suggesting that mature NBs could be sites of intranuclear proteolysis and opening new insights into NB alterations found in viral infections or transformation.
Figures













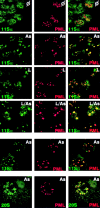
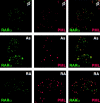

References
-
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RARα fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. - PubMed
-
- Freemont P.S. RING for destruction? Curr. Biol. 2000;10:R84–R87. - PubMed
-
- Wang Z.-G., Ruggero D., Ronchetti S., Zhong S., Gaboli M., Rivi R., Pandolfi P.P. PML is essential for multiple apoptotic pathways. Nat. Genet. 1998;20:266–272. - PubMed
-
- Wang Z.G., Delva L., Gaboli M., Rivi R., Giorgio M., Cordon-Cardo C., Grosveld F., Pandolfi P.P. Role of PML in cell growth and the retinoic acid pathway. Science. 1998;279:1547–1551. - PubMed
-
- Quignon F., de Bels F., Koken M., Feunteun J., Ameisen J.-C., de Thé H. PML induces a caspase-independent cell death process. Nat. Genet. 1998;20:259–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

