A transcription function for the T cell-specific adapter (TSAd) protein in T cells: critical role of the TSAd Src homology 2 domain
- PMID: 11413197
- PMCID: PMC2193301
- DOI: 10.1084/jem.193.12.1425
A transcription function for the T cell-specific adapter (TSAd) protein in T cells: critical role of the TSAd Src homology 2 domain
Abstract
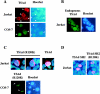
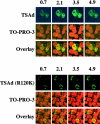
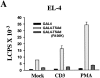
T cell-specific adapter (TSAd) protein is an Src homology 2 (SH2) domain-containing adapter molecule implicated in T cell receptor for antigen (TCR)-mediated interleukin 2 (IL-2) secretion in T cells. Here, we demonstrate that a substantial fraction of TSAd is found in the T cell nucleus. Nuclear import of TSAd is an active process that depends on TSAd SH2 domain recognition of a phosphotyrosine-containing ligand. Importantly, we show that TSAd can act as a potent transcriptional activator in T cells. Furthermore, the TSAd SH2 domain appears to be essential for this transcription-activating function independent of its role in nuclear import. Biochemical analyses suggest that a single TSAd SH2 domain ligand of 95-100 kD may be involved in these processes. Consistent with a role as a transcription activator, cotransfection of TSAd with an IL-2 promoter-reporter gene construct results in a considerable upregulation of IL-2 promoter activity. Further, we show that this augmentation requires a functional TSAd SH2 domain. However, TSAd does not appear to modulate the activity of the major recognized IL-2 gene transcription factors, nuclear factor kappaB (NF-kappaB), nuclear factor of activated T cells (NFAT), or activator protein 1 (AP-1). These findings point to the function of TSAd as a novel transcription-regulatory protein in T cells and illustrate the importance of the TSAd SH2 domain in this role.
Figures




References
-
- Clements J.L, Boerth N.J., Lee J.R., Koretzky G.A. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Ann. Rev. Immunol. 1999;17:89–108. - PubMed
-
- Spurkland A., Brinchmann J.E., Markussen G., Pedeutou F., Munthe E., Lea T., Vartdal F., Aasheim H.-C. Molecular cloning of a T cell-specific adapter protein (TSAd) containing an Src homology (SH) 2 domain and putative SH3 and phosphotyrosine binding sites. J. Biol. Chem. 1998;273:4539–4546. - PubMed
-
- Sundvold V., Torgersen K.M., Post N.H., Marti F., King P.D., Rottingen J.A., Spurkland A., Lea T. T cell-specific adapter protein inhibits T cell activation by modulating Lck activity. J. Immunol. 2000;165:2927–2931. - PubMed
-
- Choi Y.B., Kim C.K., Yun Y. Lad, an adapter protein interacting with the SH2 domain of p56lck, is required for T cell activation. J. Immunol. 1999;163:5242–5249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

