Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2
- PMID: 11413312
- PMCID: PMC114368
- DOI: 10.1128/JVI.75.14.6450-6459.2001
Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2
Abstract
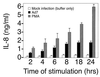
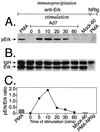
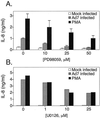
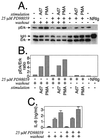
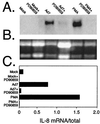
Infection with adenovirus serotype 7 (Ad7) frequently causes lower respiratory pneumonia and is associated with severe lung inflammation and neutrophil infiltration. Earlier studies indicated release of proinflammatory cytokines, specifically interleukin-8 (IL-8), by pulmonary epithelial cells following infection by Ad7. However, the mechanism of IL-8 induction by Ad7 is unclear. We have explored the role of the Ras/Raf/MEK/Erk pathway in the Ad7-associated induction of IL-8 using a model system of A549 epithelial cells. We found that Ad7 infection induced a rapid activation of epithelial cell-derived Erk. The MEK-specific inhibitors PD98059 and U0126 blocked Erk activation and release of IL-8 following infection with Ad7. Treatment with PD98059 is cytostatic and not cytotoxic, as treated cells regain the ability to phosphorylate Erk and secrete IL-8 after removal of the drug. The expression of a mutated form of Ras in A549 epithelial cells blocked the induction of IL-8 promoter activity, and MEK inhibitor blocked induction of IL-8 mRNA. These results suggest that the Ras/Raf/MEK/Erk pathway is necessary for the Ad7 induction of IL-8 and that induction occurs at the level of transcription. Further, the kinetics of Erk activation and IL-8 induction suggest that an early viral event, such as receptor binding, may be responsible for the observed inflammatory response.
Figures



References
-
- Abbandanzo S L, English C K, Kagan E, McPherson R A. Fatal adenovirus pneumonia in a newborn identified by electron microscopy and in situ hybridization. Arch Pathol Lab Med. 1989;113:1349–1353. - PubMed
-
- Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Baggiolini M, Dewal B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;33:97–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

