Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia
- PMID: 11413325
- PMCID: PMC114381
- DOI: 10.1128/JVI.75.14.6572-6583.2001
Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia
Abstract
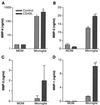
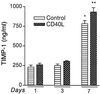
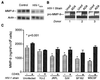
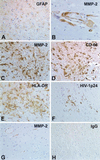
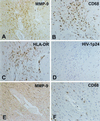
The pathogenesis of human immunodeficiency virus type 1 (HIV-1)-associated dementia (HAD) is mediated mainly by mononuclear phagocyte (MP) secretory products and their interactions with neural cells. Viral infection and MP immune activation may affect leukocyte entry into the brain. One factor that influences central nervous system (CNS) monocyte migration is matrix metalloproteinases (MMPs). In the CNS, MMPs are synthesized by resident glial cells and affect the integrity of the neuropil extracellular matrix (ECM). To ascertain how MMPs influence HAD pathogenesis, we studied their secretion following MP differentiation, viral infection, and cellular activation. HIV-1-infected and/or immune-activated monocyte-derived macrophages (MDM) and human fetal microglia were examined for production of MMP-1, -2, -3, and -9. MMP expression increased significantly with MP differentiation. Microglia secreted high levels of MMPs de novo that were further elevated following CD40 ligand-mediated cell activation. Surprisingly, HIV-1 infection of MDM led to the down-regulation of MMP-9. In encephalitic brain tissue, MMPs were expressed within perivascular and parenchymal MP, multinucleated giant cells, and microglial nodules. These data suggest that MMP production in MP is dependent on cell type, differentiation, activation, and/or viral infection. Regulation of MMP expression by these factors may contribute to neuropil ECM degradation and leukocyte migration during HAD.
Figures



References
-
- Belichenko P V, Miklossy J, Celio M R. HIV-I induced destruction of neocortical extracellular matrix components in AIDS victims. Neurobiol Dis. 1997;4:301–310. - PubMed
-
- Biggs T E, Cooke S J, Barton C H, Harris M P, Saksela K, Mann D A. Induction of activator protein 1 (AP-1) in macrophages by human immunodeficiency virus type-1 NEF is a cell-type-specific response that requires both hck and MAPK signaling events. J Mol Biol. 1999;290:21–35. - PubMed
-
- Bignami A, LeBlanc A, Perides G. A role for extracellular matrix degradation and matrix metalloproteinases in senile dementia? Acta Neuropathol. 1994;87:308–312. - PubMed
-
- Birkedal-Hansen H. Role of inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28:500–510. - PubMed
-
- Bukrinsky M I, Nottet H S L M, Schmidtmayerova H, Dubrovsky L, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

