Site-specific recognition of a 70-base-pair element containing d(GA)(n) repeats mediates bithoraxoid polycomb group response element-dependent silencing
- PMID: 11416132
- PMCID: PMC87112
- DOI: 10.1128/MCB.21.14.4528-4543.2001
Site-specific recognition of a 70-base-pair element containing d(GA)(n) repeats mediates bithoraxoid polycomb group response element-dependent silencing
Abstract
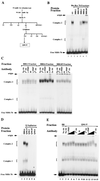
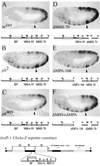
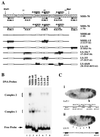
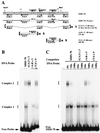
Polycomb group proteins act through Polycomb group response elements (PREs) to maintain silencing at homeotic loci. The minimal 1.5-kb bithoraxoid (bxd) PRE contains a region required for pairing-sensitive repression and flanking regions required for maintenance of embryonic silencing. Little is known about the identity of specific sequences necessary for function of the flanking regions. Using gel mobility shift analysis, we identify DNA binding activities that interact specifically with a multipartite 70-bp fragment (MHS-70) downstream of the pairing-sensitive sequence. Deletion of MHS-70 in the context of a 5.1-kb bxd Polycomb group response element derepresses maintenance of silencing in embryos. A partially purified binding activity requires multiple, nonoverlapping d(GA)(3) repeats for MHS-70 binding in vitro. Mutation of d(GA)(3) repeats within MHS-70 in the context of the 5.1-kb bxd PRE destabilizes maintenance of silencing in a subset of cells in vivo but gives weaker derepression than deletion of MHS-70. These results suggest that d(GA)(3) repeats are important for silencing but that other sequences within MHS-70 also contribute to silencing. Antibody supershift assays and Western analyses show that distinct isoforms of Polyhomeotic and two proteins that recognize d(GA)(3) repeats, the TRL/GAGA factor and Pipsqueak (Psq), are present in the MHS-70 binding activity. Mutations in Trl and psq enhance homeotic phenotypes of ph, indicating that TRL/GAGA factor and Psq are enhancers of Polycomb which have sequence-specific DNA binding activity. These studies demonstrate that site-specific recognition of the bxd PRE by d(GA)(n) repeat binding activities mediates PcG-dependent silencing.
Figures



References
-
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. - PubMed
-
- Bender W, Akam M, Karch F, Beachy P A, Peifer M, Spierer P, Lewis E B, Hogness D S. Molecular genetics of the Bithorax complex in Drosophila melanogaster. Science. 1983;221:23–29. - PubMed
-
- Bhat K M, Farkas G, Karch F, Gyurkovics H, Gausz J, Schedl P. The GAGA factor is required in the early Drosophila embryo not only for transcriptional regulation but also for nuclear division. Development. 1996;122:1513–1522. - PubMed
-
- Biggin M D, Tjian R. Transcription factors that activate the Ultrabithorax promotor in developmentally staged extracts. Cell. 1988;53:699–711. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
