Protein misfolding and temperature up-shift cause G1 arrest via a common mechanism dependent on heat shock factor in Saccharomycescerevisiae
- PMID: 11416208
- PMCID: PMC34665
- DOI: 10.1073/pnas.121172998
Protein misfolding and temperature up-shift cause G1 arrest via a common mechanism dependent on heat shock factor in Saccharomycescerevisiae
Abstract
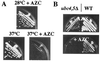
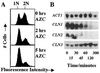
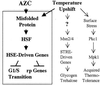
Accumulation of misfolded proteins in the cell at high temperature may cause entry into a nonproliferating, heat-shocked state. The imino acid analog azetidine 2-carboxylic acid (AZC) is incorporated into cellular protein competitively with proline and can misfold proteins into which it is incorporated. AZC addition to budding yeast cells at concentrations sufficient to inhibit proliferation selectively activates heat shock factor (HSF). We find that AZC treatment fails to cause accumulation of glycogen and trehalose (Msn2/4-dependent processes) or to induce thermotolerance (a protein kinase C-dependent process). However, AZC-arrested cells can accumulate glycogen and trehalose and can acquire thermotolerance in response to a subsequent heat shock. We find that AZC treatment arrests cells in a viable state and that this arrest is reversible. We find that cells at high temperature or cells deficient in the ubiquitin-conjugating enzymes Ubc4 and Ubc5 are hypersensitive to AZC-induced proliferation arrest. We find that AZC treatment mimics temperature up-shift in arresting cells in G1 and represses expression of CLN1 and CLN2. Mutants with reduced G1 cyclin-Cdc28 activity are hypersensitive to AZC-induced proliferation arrest. Expression of the hyperstable Cln3-2 protein prevents G1 arrest upon AZC treatment and temperature up-shift. Finally, we find that the EXA3-1 mutation, encoding a defective HSF, prevents efficient G1 arrest in response to both temperature up-shift and AZC treatment. We conclude that nontoxic levels of misfolded proteins (induced by AZC treatment or by high temperature) selectively activate HSF, which is required for subsequent G1 arrest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

