Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore
- PMID: 11418573
- PMCID: PMC95322
- DOI: 10.1128/JB.183.14.4317-4322.2001
Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore
Abstract
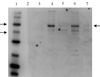
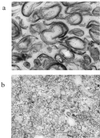
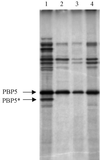
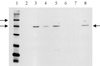
The GerAA, -AB, and -AC proteins of the Bacillus subtilis spore are required for the germination response to L-alanine as the sole germinant. They are likely to encode the components of the germination apparatus that respond directly to this germinant, mediating the spore's response; multiple homologues of the gerA genes are found in every spore former so far examined. The gerA operon is expressed in the forespore, and the level of expression of the operon appears to be low. The GerA proteins are predicted to be membrane associated. In an attempt to localize GerA proteins, spores of B. subtilis were broken and fractionated to give integument, membrane, and soluble fractions. Using antibodies that detect Ger proteins specifically, as confirmed by the analysis of strains lacking GerA and the related GerB proteins, the GerAA protein and the GerAC+GerBC protein homologues were localized to the membrane fraction of fragmented spores. The spore-specific penicillin-binding protein PBP5*, a marker for the outer forespore membrane, was absent from this fraction. Extraction of spores to remove coat layers did not release the GerAC or AA protein from the spores. Both experimental approaches suggest that GerAA and GerAC proteins are located in the inner spore membrane, which forms a boundary around the cellular compartment of the spore. The results provide support for a model of germination in which, in order to initiate germination, germinant has to permeate the coat and cortex of the spore and bind to a germination receptor located in the inner membrane.
Figures


References
-
- Chambers S P, Prior S E, Barstow D A, Minton N. The pMTL nic-cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988;68:139–149. - PubMed
-
- Corfe B M, Moir A, Popham D, Setlow P. Analysis of the expression and regulation of the gerB spore germination operon of Bacillus subtilis 168. Microbiology. 1994;140:3079–3083. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

