Spermatogenesis in Bclw-deficient mice
- PMID: 11420255
- PMCID: PMC3049812
- DOI: 10.1095/biolreprod65.1.318
Spermatogenesis in Bclw-deficient mice
Abstract
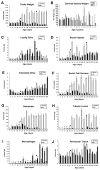
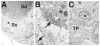
Bclw is a death-protecting member of the Bcl2 family of apoptosis-regulating proteins. Mice that are mutant for Bclw display progressive and nearly complete testicular degeneration. We performed a morphometric evaluation of testicular histopathology in Bclw-deficient male mice between 9 days postnatal (p9) through 1 yr of age. Germ cell loss began by p22, with only few germ cells remaining beyond 7 mo of age. A complete block to elongated spermatid development at step 13 occurred during the first wave of spermatogenesis, whereas other types of germ cells were lost sporadically. Depletion of Sertoli cells commenced between p20 and p23 and continued until 1 yr of age, when few, if any, Sertoli cells remained. Mitochondria appeared to be swollen and the cytoplasm dense by electron microscopy, but degenerating Bclw-deficient Sertoli cells failed to display classical features of apoptosis, such as chromatin condensation and nuclear fragmentation. Macrophages entered seminiferous tubules and formed foreign-body giant cells that engulfed and phagocytosed the degenerated Sertoli cells. Leydig cell hyperplasia was evident between 3 and 5 mo of age. However, beginning at 7 mo of age, Leydig cells underwent apoptosis, with dead cells being phagocytosed by macrophages. The aforementioned cell losses culminated in a testis-containing vasculature, intertubular phagocytic cells, and peritubular cell "ghosts." An RNA in situ hybridization study indicates that Bclw is expressed in Sertoli cells in the adult mouse testis. Consequently, the diploid germ cell death may be an indirect effect of defective Sertoli cell function. Western analysis was used to confirm that Bclw is not expressed in spermatids; thus, loss of this cell type most likely results from defective Sertoli cell function. Because Bclw does not appear to be expressed in Leydig cells, loss of Leydig cells in Bclw-deficient mice may result from depletion of Sertoli cells. Bclw-deficient mice serve as a unique model to study homeostasis of cell populations in the testis.
Figures







References
-
- Capel B. The battle of the sexes. Mech Dev. 2000;92:89–103. - PubMed
-
- Vergouwen RPFA, Jacobs SGPM, Huiskamp R, Davids JAG, de Rooij DG. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–243. - PubMed
-
- Steinberger A, Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol Reprod. 1971;4:84–87. - PubMed
-
- Russell LD, Bartke A, Goh JC. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat. 1989;184:179–189. - PubMed
-
- Ross A, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–255. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

