Influence of cirrhosis on lamotrigine pharmacokinetics
- PMID: 11421997
- PMCID: PMC2014475
- DOI: 10.1046/j.1365-2125.2001.01389.x
Influence of cirrhosis on lamotrigine pharmacokinetics
Abstract
Aims: Lamotrigine, an antiepileptic drug, is cleared from the systemic circulation mainly by glucuronidation. The possibility of changes in the pharmacokinetics of lamotrigine in plasma owing to hepatic dysfunction has been evaluated.
Methods: Thirty-six subjects, including 24 patients with various degrees of liver cirrhosis and 12 healthy volunteers received a single 100 mg dose of lamotrgine. Blood samples were taken for 7 days in all subjects, except nine with severe cirrhosis, who had a 29 day blood sampling period.
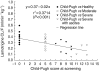
Results: The pharmacokinetics of lamotrigine were comparable between the patients with moderate cirrhosis (corresponding to Child-Pugh grade A) and the healthy subjects. Plasma oral clearance mean ratios (90% confidence interval) in patients with severe cirrhosis without or with ascites (corresponding, respectively, to Child-Pugh grade B and C) to healthy subjects were, respectively, 60% (44%, 83%) and 36% (25%, 52%). Plasma half-life mean ratios (90% confidence interval) in these two patient groups to healthy subjects were, respectively, 204% (149%, 278%) and 287% (202%, 408%).
Conclusions: Lamotrigine administered as a single oral dose of 100 mg was well tolerated in all groups. Initial, escalation and maintenance doses should generally be reduced by approximately 50 or 75% in patients with Child-Pugh Grade B or C cirrhosis. Escalation and maintenance doses should be adjusted according to clinical response.
Figures

References
-
- Goa KL, Ross SR, Chrisp P. Lamotrigine: a review of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1993;46:1–25. - PubMed
-
- Yuen AWC, Peck AW. Lamotrigine pharmacokinetics: oral and i.v. infusion in man. Br J Clin Pharmacol. 1988;26:242.
-
- Kroemer H, Klotz U. Glucuronidation of drugs. A reevaluation of the pharmacological significance of the conjugates and modulating factors. Clin Pharmacokinet. 1992;23(4):292–310. - PubMed
-
- George J, Byth K. Influence of clinicopathological variables on CYP protein expression in human liver. J Gastroenterol Hepatol. 1996;11:33–39. - PubMed
-
- Pugh RNH, Murray-Lion IM, Dawson JL, Pietroni MC, Williams R. Transsection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:289–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

