Skeletal muscle necrosis and regeneration after injection of Thalassophryne nattereri (niquim) fish venom in mice
- PMID: 11422541
- PMCID: PMC2517697
- DOI: 10.1046/j.1365-2613.2001.00181.x
Skeletal muscle necrosis and regeneration after injection of Thalassophryne nattereri (niquim) fish venom in mice
Abstract
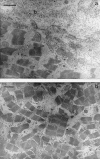
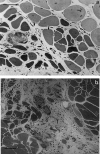
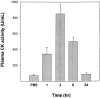
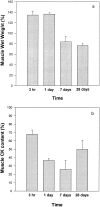
Stings by Thalassophryne nattereri are responsible for envenomation of fishermen in north-eastern Brazil. Its venom induces prominent local tissue damage, characterized by pain, oedema and necrosis. The pathogenesis of acute muscle damage induced by T. nattereri venom was studied in mice. Intramuscular injection induced myonecrosis within the first hours. Some muscle cells presented a hypercontracted morphology, but most necrotic fibres were not hypercontracted, being instead characterized by a disorganization of myofibrils, with Z line loss, mitochondrial swelling and sarcolemmal disruption. In addition, thrombosis was observed histologically in venules and veins, together with vascular congestion and stasis, evidenced by intravital microscopy. Venom induced a rapid increment in serum creatine kinase (CK) levels, concomitant with a reduction in gastrocnemius muscle CK activity, whereas no increments in muscle lactic acid were detected. A rapid cytolytic effect was induced by the venom on C2C12 murine myoblasts in culture. The inflammatory reaction in affected muscle was characterized by oedema and scarce cellular infiltrate of polymorphonuclear leucocytes and macrophages, with a consequent delay in the removal of necrotic material. Skeletal muscle regeneration was partially impaired, as evidenced by the presence of regenerating fibres of variable size and by the increase of fibrotic tissue in endomysium and perimysium. It is suggested that T. nattereri venom affects muscle fibres by a direct cytotoxic effect, and that the vascular alterations described preclude a successful regenerative process.
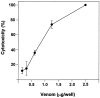
Figures



Similar articles
-
Characterisation of local inflammatory response induced by Thalassophryne nattereri fish venom in a mouse model of tissue injury.Toxicon. 2003 Oct;42(5):499-507. doi: 10.1016/s0041-0101(03)00228-9. Toxicon. 2003. PMID: 14529731
-
Hemostatic effects induced by Thalassophryne nattereri fish venom: a model of endothelium-mediated blood flow impairment.Toxicon. 2002 Aug;40(8):1141-147. doi: 10.1016/s0041-0101(02)00114-9. Toxicon. 2002. PMID: 12165317
-
Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom.Muscle Nerve. 2003 Oct;28(4):449-59. doi: 10.1002/mus.10453. Muscle Nerve. 2003. PMID: 14506717
-
Why is Skeletal Muscle Regeneration Impaired after Myonecrosis Induced by Viperid Snake Venoms?Toxins (Basel). 2018 May 1;10(5):182. doi: 10.3390/toxins10050182. Toxins (Basel). 2018. PMID: 29723952 Free PMC article. Review.
-
Angiotensin converting enzymes in fish venom.Toxicon. 2017 Jun 1;131:63-67. doi: 10.1016/j.toxicon.2017.03.003. Epub 2017 Mar 8. Toxicon. 2017. PMID: 28284848 Review.
Cited by
-
Cell death, clearance and immunity in the skeletal muscle.Cell Death Differ. 2016 Jun;23(6):927-37. doi: 10.1038/cdd.2015.171. Epub 2016 Feb 12. Cell Death Differ. 2016. PMID: 26868912 Free PMC article. Review.
-
Bioactive components in fish venoms.Toxins (Basel). 2015 Apr 30;7(5):1497-531. doi: 10.3390/toxins7051497. Toxins (Basel). 2015. PMID: 25941767 Free PMC article. Review.
-
Biotoxins in muscle regeneration research.J Muscle Res Cell Motil. 2019 Dec;40(3-4):291-297. doi: 10.1007/s10974-019-09548-4. Epub 2019 Jul 29. J Muscle Res Cell Motil. 2019. PMID: 31359301 Review.
-
Delayed local inflammatory response induced by Thalassophryne nattereri venom is related to extracellular matrix degradation.Int J Exp Pathol. 2009 Feb;90(1):34-43. doi: 10.1111/j.1365-2613.2008.00603.x. Int J Exp Pathol. 2009. PMID: 19200249 Free PMC article.
-
Histopathological characterization of skin and muscle lesions induced by lionfish (Pterois volitans) venom in a murine experimental model.J Venom Anim Toxins Incl Trop Dis. 2025 Jan 17;31:e20240050. doi: 10.1590/1678-9199-JVATITD-2024-0050. eCollection 2025. J Venom Anim Toxins Incl Trop Dis. 2025. PMID: 39877151 Free PMC article.
References
-
- Allbrook D. Skeletal muscle regeneration. Muscle & Nerve. 1981;4:234–245. - PubMed
-
- Auger MJ, Ross JA. The biology of the macrophage. In: Lewis CE, MCGEE JOD, editors. The Macrophage. Oxford: IRL press; 1992. pp. 2–74.
-
- Auto HF. Acidentes por peixes peçonhentos Thalassophryne(Niquim), consideraçoes em torno de 32 casos. RevEscCiencias Médicas Alagoas. 1992;5:35–36.
-
- Azevedo-Marques MM, Cupo P, Coimbra TM, Hering SE, Rossi MA, Laure CJ. Myonecrosis, myoglobinuria and acute renal failure induced by South American rattlesnake(Crotalus durissus terrificus) envenomation in Brazil. Toxicon. 1985;23:631–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

