Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol
- PMID: 11425711
- PMCID: PMC92970
- DOI: 10.1128/AEM.67.7.2982-2992.2001
Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol
Abstract
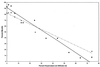
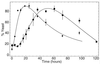
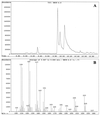
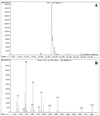
The inoculum size effect in the dimorphic fungus Candida albicans results from production of an extracellular quorum-sensing molecule (QSM). This molecule prevents mycelial development in both a growth morphology assay and a differentiation assay using three chemically distinct triggers for germ tube formation (GTF): L-proline, N-acetylglucosamine, and serum (either pig or fetal bovine). In all cases, the presence of QSM prevents the yeast-to-mycelium conversion, resulting in actively budding yeasts without influencing cellular growth rates. QSM exhibits general cross-reactivity within C. albicans in that supernatants from strain A72 are active on five other strains of C. albicans and vice versa. The QSM excreted by C. albicans is farnesol (C(15)H(26)O; molecular weight, 222.37). QSM is extracellular, and is produced continuously during growth and over a temperature range from 23 to 43 degrees C, in amounts roughly proportional to the CFU/milliliter. Production is not dependent on the type of carbon source nor nitrogen source or on the chemical nature of the growth medium. Both commercial mixed isomer and (E,E)-farnesol exhibited QSM activity (the ability to prevent GTF) at a level sufficient to account for all the QSM activity present in C. albicans supernatants, i.e., 50% GTF at ca. 30 to 35 microM. Nerolidol was ca. two times less active than farnesol. Neither geraniol (C(10)), geranylgeraniol (C(20)), nor farnesyl pyrophosphate had any QSM activity.
Figures



References
-
- Atlas R M. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1993.
-
- Bard M, Albrecht M R, Gupta N, Guynn C J, Stilwell W. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids. 1988;23:534–538. - PubMed
-
- Brown A J P, Gow N A R. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. - PubMed
-
- Cha C, Gao P, Chen Y-C, Shaw P D, Farrand S K. Production of acyl-homoserine lactone quorum sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

