The presynaptic function of mouse cochlear inner hair cells during development of hearing
- PMID: 11425887
- PMCID: PMC6762371
- DOI: 10.1523/JNEUROSCI.21-13-04593.2001
The presynaptic function of mouse cochlear inner hair cells during development of hearing
Abstract
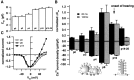
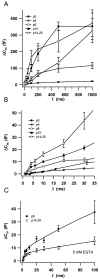
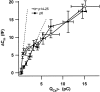
Before mice start to hear at approximately postnatal day 10, their cochlear inner hair cells (IHCs) spontaneously generate Ca(2+) action potentials. Therefore, immature IHCs could stimulate the auditory pathway, provided that they were already competent for transmitter release. Here, we combined patch-clamp capacitance measurements and fluorimetric [Ca(2+)](i) recordings to study the presynaptic function of IHCs during cochlear maturation. Ca(2+)-dependent exocytosis and subsequent endocytic membrane retrieval were already observed near the date of birth. Ca(2+) action potentials triggered exocytosis in immature IHCs, which probably activates the auditory pathway before it becomes responsive to sound. IHCs underwent profound changes in Ca(2+)-channel expression and secretion during their postnatal development. Ca(2+)-channel expression increased toward the end of the first week, providing for large secretory responses during this period and thereafter declined to reach mature levels. The efficacy whereby Ca(2+) influx triggers exocytosis increased toward maturation, such that vesicle fusion caused by a given Ca(2+) current occurred faster in mature IHCs. The observed changes in Ca(2+)-channel expression and synaptic efficacy probably reflected the ongoing synaptogenesis in IHCs that had been described previously in morphological studies.
Figures


References
-
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. - PubMed
-
- Ehret G. Behavioural studies on auditory development in mammals in relation to higher nervous system functioning. Acta Otolaryngol [Suppl] 1985;421:31–40. - PubMed
-
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O'Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. - PubMed
-
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
