Activation of the nuclear factor-kappaB is a key event in brain tolerance
- PMID: 11425894
- PMCID: PMC6762345
- DOI: 10.1523/JNEUROSCI.21-13-04668.2001
Activation of the nuclear factor-kappaB is a key event in brain tolerance
Abstract
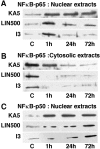
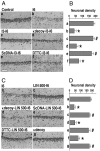
The transcription factor nuclear factor-kappaB (NFkappaB) is an ubiquitously expressed inducible regulator of a broad range of genes and plays a pivotal role in cell death and survival pathways. Three models of brain tolerance (ischemic, epileptic, and polyunsaturated fatty acid-induced preconditioning), known to confer resistance to neurons against ischemia or status epilepticus, were used to determine whether NFkappaB mediated the late preconditioning. A sublethal 3 min ischemia, a dose of 5 mg/kg kainic acid (KA5) or 500 nmol of linolenic acid (LIN500) led to a rapid increase of NFkappaB DNA-binding activity and nuclear translocation of p65 and p50 subunits of NFkappaB in neurons. Pretreatment with the NFkappaB inhibitor diethyldithiocarbamate or kappaB decoy DNA blocked the increased DNA-binding activity and the nuclear translocation of NFkappaB and abolished the neuroprotective effects of different delayed preconditionings against severe ischemia or epilepsy. The inhibition of NFkappaB observed in rats preconditioned with 3 min ischemia, KA5 or LIN500 treatments compared with ischemic or epileptic controls was correlated with the prevention of the inducible degradation of the inhibitory protein IkappaBalpha. Preconditioning probably inhibits the activation of NFkappaB by interfering with a pathway that leads to the direct transcriptional activation of IkappaBalpha by NFkappaB itself. The present work provides evidence that activation of NFkappaB is a crucial step in the signal transduction pathway that underlies the development of brain tolerance and may open new strategies in the prevention of cerebral diseases, such as ischemia or epilepsy.
Figures




References
-
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. - PubMed
-
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. - PubMed
-
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. - PMC - PubMed
-
- Blondeau N, Plamondon H, Richelme C, Heurteaux C, Lazdunski M. KATP channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience. 2000;100:465–474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
