Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons
- PMID: 11425919
- PMCID: PMC6762358
- DOI: 10.1523/JNEUROSCI.21-13-04915.2001
Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons
Abstract
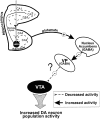
Several studies have shown that the mesolimbic dopamine (DA) system is strongly influenced by the ventral subiculum (vSub) of the hippocampus. To examine whether this occurs by activation of DA neuron firing, the effects of chemical stimulation of the vSub on ventral tegmental area (VTA) DA neuron activity were examined using extracellular single-unit recordings. Infusions of NMDA into the vSub increased the number of spontaneously firing DA cells recorded per electrode track, while having no effect on firing rate or burst firing. This response was abolished by intranucleus accumbens (NAc) infusions of the glutamate receptor antagonist kynurenic acid. This effect did not involve the prefrontal cortex, because infusions of tetrodotoxin into the prefrontal cortex did not affect the increase in spontaneously active DA cells. Infusions of either kynurenic acid into the NAc or tetrodotoxin into the vSub decreased the firing rate and burst firing of DA neurons without altering the number of spontaneously active DA neurons. These data show that glutamatergic afferents from the vSub to the NAc exert a potent excitatory effect on VTA DA neurons, influencing both DA neuron population activity and the regulation of the firing properties of these neurons. As a result, dysfunctions in hippocampal circuitries may contribute to the hyperexcitable state of the DA system that is present in schizophrenia.
Figures




References
-
- Bardgett ME, Henry JD. Locomotor activity and accumbens fos expression driven by ventral hippocampal stimulation require D1 and D2 receptors. Neuroscience. 1999;94:59–70. - PubMed
-
- Blaha CD, Allen LF, Das S, Ingles WL, Latimet MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. - PMC - PubMed
-
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;5:902–911. - PubMed
-
- Bogerts B. Recent advances in the neuropathology of schizophrenia. Schizophr Bull. 1993;19:431–445. - PubMed
-
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
