Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips
- PMID: 11427565
- PMCID: PMC88181
- DOI: 10.1128/JCM.39.7.2531-2540.2001
Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips
Abstract
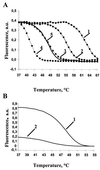
Three new molecular approaches were developed to identify drug-resistant strains of Mycobacterium tuberculosis using biochips with oligonucleotides immobilized in polyacrylamide gel pads. These approaches are significantly faster than traditional bacteriological methods. All three approaches-hybridization, PCR, and ligase detection reaction--were designed to analyze an 81-bp fragment of the gene rpoB encoding the beta-subunit of RNA polymerase, where most known mutations of rifampin resistance are located. The call set for hybridization analysis consisted of 42 immobilized oligonucleotides and enabled us to identify 30 mutant variants of the rpoB gene within 24 h. These variants are found in 95% of all mutants whose rifampin resistance is caused by mutations in the 81-bp fragment. Using the second approach, allele-specific on-chip PCR, it was possible to directly identify mutations in clinical samples within 1.5 h. The third approach, on-chip ligase detection reaction, was sensitive enough to reveal rifampin-resistant strains in a model mixture containing 1% of resistant and 99% of susceptible bacteria. This level of sensitivity is comparable to that from the determination of M. tuberculosis drug resistance by using standard bacteriological tests.
Figures


References
-
- Bocharov P P. Series criterion based on median. In: Pechinkin A B, editor. Mathematical statistics. 1994. p. 164. RUDN, Moscow, Russia.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

