Neurotensin-deficient mice show altered responses to antipsychotic drugs
- PMID: 11427716
- PMCID: PMC35465
- DOI: 10.1073/pnas.141042198
Neurotensin-deficient mice show altered responses to antipsychotic drugs
Abstract
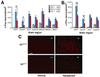
The peptide transmitter neurotensin (NT) exerts diverse neurochemical effects that resemble those seen after acute administration of antipsychotic drugs (APDs). These drugs also induce NT expression in the striatum; this and other convergent findings have led to the suggestion that NT may mediate some APD effects. Here, we demonstrate that the ability of the typical APD haloperidol to induce Fos expression in the dorsolateral striatum is markedly attenuated in NT-null mutant mice. The induction of Fos and NT in the dorsolateral striatum in response to typical, but not atypical, APDs has led to the hypothesis that the increased expression of these proteins is mechanistically related to the production of extrapyramidal side effects (EPS). However, we found that catalepsy, which is thought to reflect the EPS of typical APDs, is unaffected in NT-null mutant mice, suggesting that NT does not contribute to the generation of EPS. We conclude that NT is required for haloperidol-elicited activation of a specific population of striatal neurons but not haloperidol-induced catalepsy. These results are consistent with the hypothesis that endogenous NT mediates a specific subset of APD actions.
Figures


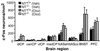

References
-
- Deutch A Y, Moghaddam B, Innis R B, Krystal J H, Aghajanian G K, Bunney B S, Charney D S. Schizophr Res. 1991;4:121–156. - PubMed
-
- Meltzer H Y, Deutch A Y. In: Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Seigel G J, Agranoff B W, Albers R W, Fisher S K, Ehler M D, editors. New York: Lippincott-Raven; 1999. pp. 1053–1072.
-
- Creese I, Burt D R, Snyder S H. Science. 1976;192:481–483. - PubMed
-
- Seeman P. Synapse. 1987;1:133–152. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

