Different modes of hippocampal plasticity in response to estrogen in young and aged female rats
- PMID: 11427724
- PMCID: PMC35469
- DOI: 10.1073/pnas.141215898
Different modes of hippocampal plasticity in response to estrogen in young and aged female rats
Abstract
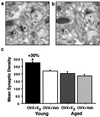
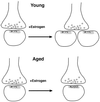
Estrogen regulates hippocampal dendritic spine density and synapse number in an N-methyl-D-aspartate (NMDA) receptor-dependent manner, and these effects may be of particular importance in the context of age-related changes in endocrine status. We investigated estrogen's effects on axospinous synapse density and the synaptic distribution of the NMDA receptor subunit, NR1, within the context of aging. Although estrogen induced an increase in axospinous synapse density in young animals, it did not alter the synaptic representation of NR1, in that the amount of NR1 per synapse was equivalent across groups. Estrogen replacement in aged female rats failed to increase axospinous synapse density; however, estrogen up-regulated synaptic NR1 compared with aged animals with no estrogen. Therefore, the young and aged hippocampi react differently to estrogen replacement, with the aged animals unable to mount a plasticity response generating additional synapses, yet responsive to estrogen with respect to additional NMDA receptor content per synapse. These findings have important implications for estrogen replacement therapy in the context of aging.
Figures


References
-
- Lamberts S W J, van den Beld A W, van der Lely A-J. Science. 1997;278:419–424. - PubMed
-
- Fink G. Sci Prog. 1986;70:403–423. - PubMed
-
- Woolley C S. Horm Behav. 1998;34:140–148. - PubMed
-
- Sherwin B B. Neurology. 1997;48:S21–S26. - PubMed
-
- Berry B, McMahan R, Gallagher M. Behav Neurosci. 1997;111:267–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

