Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism
- PMID: 11427728
- PMCID: PMC35419
- DOI: 10.1073/pnas.141224398
Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism
Abstract
The Ser/Thr kinase Raf-1 is a protooncogene product that is a central component in many signaling pathways involved in normal cell growth and oncogenic transformation. Upon activation, Raf-1 phosphorylates mitogen-activated protein kinase kinase (MEK), which in turn activates mitogen-activated protein kinase/extracellular signal-regulated kinases (MAPK/ERKs), leading to the propagation of signals. Depending on specific stimuli and cellular environment, the Raf-1--MEK--ERK cascade regulates diverse cellular processes such as proliferation, differentiation, and apoptosis. Here, we describe a MEK--ERK-independent prosurvival function of Raf-1. We found that Raf-1 interacts with the proapoptotic, stress-activated protein kinase ASK1 (apoptosis signal-regulating kinase 1) in vitro and in vivo. Deletion analysis localized the Raf-1 binding site to the N-terminal regulatory fragment of ASK1. This interaction allows Raf-1 to act independently of the MEK--ERK pathway to inhibit apoptosis. Furthermore, catalytically inactive forms of Raf-1 can mimic the wild-type effect, raising the possibility of a kinase-independent function of Raf-1. Thus, Raf-1 may promote cell survival through its protein-protein interactions in addition to its established MEK kinase function.
Figures
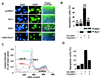



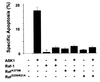
References
-
- Morrison D K, Cutler R E. Curr Opin Cell Biol. 1997;9:174–179. - PubMed
-
- Cleveland J L, Troppmair J, Packham G, Askew D S, Lloyd P, Gonzalez-Garcia M, Nunez G, Ihle J N, Rapp U R. Oncogene. 1994;9:2217–2226. - PubMed
-
- Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

