Xenopus Bcl-X(L) selectively protects Rohon-Beard neurons from metamorphic degeneration
- PMID: 11427732
- PMCID: PMC35434
- DOI: 10.1073/pnas.141226798
Xenopus Bcl-X(L) selectively protects Rohon-Beard neurons from metamorphic degeneration
Abstract
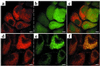
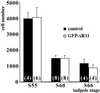
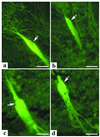
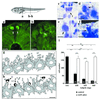
Amphibian metamorphosis involves extensive, but selective, neuronal death and turnover, thus sharing many features with mammalian postnatal development. The antiapoptotic protein Bcl-X(L) plays an important role in postnatal mammalian neuronal survival. It is therefore of interest that accumulation of the mRNA encoding the Xenopus Bcl-X(L) homologue, termed xR11, increases abruptly in the nervous system, but not in other tissues, during metamorphosis in Xenopus tadpoles. This observation raises the intriguing possibility that xR11 selectively regulates neuronal survival during postembryonic development. To investigate this hypothesis, we overexpressed xR11 in vivo as a green fluorescent protein (GFP)-xR11 fusion protein by using somatic and germinal transgenesis. Somatic gene transfer showed that the fusion protein was effective in counteracting, in a dose-dependent manner, the proapoptotic effects of coexpressed Bax. When GFP-xR11 was expressed from the neuronal beta-tubulin promoter by germinal transgenesis we observed neuronal specific expression that was maintained throughout metamorphosis and beyond, into juvenile and adult stages. Confocal microscopy showed GFP-xR11 to be exclusively localized in the mitochondria. Our findings show that GFP-xR11 significantly prolonged Rohon-Beard neuron survival up to the climax of metamorphosis, even in the regressing tadpole tail, whereas in controls these neurons disappeared in early metamorphosis. However, GFP-xR11 expression did not modify the fate of spinal cord motoneurons. The selective protection of Rohon-Beard neurons reveals cell-specific apoptotic pathways and offers approaches to further analyze programmed neuronal turnover during postembryonic development.
Figures
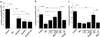

References
-
- Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. - PubMed
-
- Kerr J F, Harmon B, Searle J. J Cell Sci. 1974;14:571–585. - PubMed
-
- Gross A, McDonnell J M, Korsmeyer S J. Genes Dev. 1999;13:1899–1911. - PubMed
-
- Loeffler M, Kroemer G. Exp Cell Res. 2000;256:19–26. - PubMed
-
- Hengartner M O. Nature (London) 2000;407:770–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

