Nitric oxide- and nitric oxide donors-induced relaxation and its modulation by oxidative stress in piglet pulmonary arteries
- PMID: 11429384
- PMCID: PMC1572823
- DOI: 10.1038/sj.bjp.0704103
Nitric oxide- and nitric oxide donors-induced relaxation and its modulation by oxidative stress in piglet pulmonary arteries
Abstract
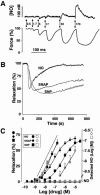
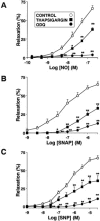
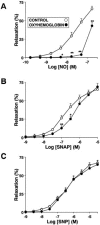
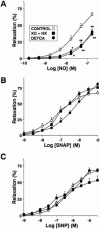
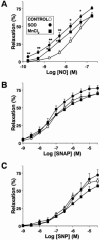
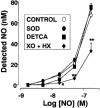
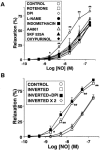
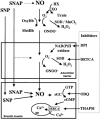
Inhaled nitric oxide (iNO) is widely used in the treatment of pulmonary hypertension while inhaled NO donors have been suggested as an alternative therapy. The differential susceptibility to inactivation by oxidative stress and oxyhaemoglobin of NO and two NO donors, sodium nitroprusside (SNP) and S-nitroso-N-acetyl-penicillamine (SNAP) were analysed in isolated endothelium-denuded pulmonary arteries from 2-week-old piglets stimulated with U46619. NO, SNAP and SNP relaxed the arteries (pIC(30)=7.73+/-0.12, 7.26+/-0.17 and 6.43+/-0.13, respectively) but NO was not detected electrochemically in the bath after the addition of SNP and only at concentrations at which SNAP produced more than 50% relaxation. The sGC inhibitor ODQ (10(-6) M) or the sarcoplasmic Ca(2+)-ATPase thapsigargin (2x10(-6) M) markedly inhibited the relaxation induced by NO, SNAP and SNP. Addition of oxyhaemoglobin (3x10(-7) M) or diethyldithiocarbamate (1 mM) markedly inhibited NO- (pIC(30)=6.88+/-0.07 and 6.92+/-0.18, respectively), weakly inhibited SNAP- and had no effect on SNP-induced relaxation. Xanthine oxidase (5 mu ml(-1)) plus hypoxanthine (10(-4) M) markedly inhibited NO- (pIC(30)=6.96+/-0.12) but not SNAP- or SNP-induced relaxation. Superoxide dismutase (SOD), MnCl(2), diphenileneiodonium and exposing the luminal surface of the rings outwards (inversion) potentiated the relaxant responses of NO (pIC(30)=8.52+/-0.16, 8.23+/-0.11, 8.01+/-0.11 and 8.20+/-0.10, respectively). However, SOD did not modify the NO detected by the electrode and had no effect on SNAP- or SNP-induced relaxation. Therefore, the kinetics and local distribution of NO release of NO donors influence the susceptibility to the scavenging effects of oxyhaemoglobin and superoxide.
Figures
Similar articles
-
Mechanisms involved in SNP-induced relaxation and [Ca+]i reduction in piglet pulmonary and systemic arteries.Br J Pharmacol. 2001 Feb;132(4):959-67. doi: 10.1038/sj.bjp.0703894. Br J Pharmacol. 2001. PMID: 11181438 Free PMC article.
-
The role of nitric oxide synthase-derived reactive oxygen species in the altered relaxation of pulmonary arteries from lambs with increased pulmonary blood flow.Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1491-7. doi: 10.1152/ajpheart.00185.2007. Epub 2007 May 18. Am J Physiol Heart Circ Physiol. 2007. PMID: 17513498 Free PMC article.
-
Postnatal maturation in nitric oxide-induced pulmonary artery relaxation involving cyclooxygenase-1 activity.Am J Physiol Lung Cell Mol Physiol. 2002 Oct;283(4):L839-48. doi: 10.1152/ajplung.00293.2001. Am J Physiol Lung Cell Mol Physiol. 2002. PMID: 12225961
-
Divergent effects of vitamin C on relaxations of rabbit aortic rings to acetylcholine and NO-donors.Br J Pharmacol. 2002 Feb;135(4):1044-50. doi: 10.1038/sj.bjp.0704541. Br J Pharmacol. 2002. PMID: 11861333 Free PMC article.
-
Involvement of protein kinase C in reduced relaxant responses to the NO/cyclic GMP pathway in piglet pulmonary arteries contracted by the thromboxane A2-mimetic U46619.Br J Pharmacol. 1997 Aug;121(7):1323-33. doi: 10.1038/sj.bjp.0701257. Br J Pharmacol. 1997. PMID: 9257910 Free PMC article.
Cited by
-
Periprocedural Management of Vein of Galen Aneurysmal Malformation Patients: An 11-Year Experience.Anesth Essays Res. 2017 Jul-Sep;11(3):630-635. doi: 10.4103/aer.AER_252_16. Anesth Essays Res. 2017. PMID: 28928561 Free PMC article.
-
Dietary copper supplements modulate aortic superoxide dismutase, nitric oxide and atherosclerosis.Int J Exp Pathol. 2005 Aug;86(4):247-55. doi: 10.1111/j.0959-9673.2005.00432.x. Int J Exp Pathol. 2005. PMID: 16045547 Free PMC article.
-
S -1-Propenylcysteine, a sulfur compound in aged garlic extract, alleviates cold-induced reduction in peripheral blood flow in rat via activation of the AMPK/eNOS/NO pathway.Exp Ther Med. 2020 Sep;20(3):2815-2821. doi: 10.3892/etm.2020.8969. Epub 2020 Jul 7. Exp Ther Med. 2020. PMID: 32765777 Free PMC article.
-
Comparative study of yeast selenium vs. sodium selenite on growth performance, nutrient digestibility, anti-inflammatory and anti-oxidative activity in weaned piglets challenged by Salmonella typhimurium.Innate Immun. 2020 May;26(4):248-258. doi: 10.1177/1753425919888566. Epub 2019 Nov 25. Innate Immun. 2020. PMID: 31766926 Free PMC article.
-
Ruthenium complexes as NO donors for vascular relaxation induction.Molecules. 2014 Jul 7;19(7):9628-54. doi: 10.3390/molecules19079628. Molecules. 2014. PMID: 25004072 Free PMC article. Review.
References
-
- ABMAN S.H., CHATFIELD B., HALL S.L., MCMURTRY I.F. Role of endothelium-derived releasing factor during the transition of the pulmonary circulation at birth. Am. J. Physiol. 1990;259:H1921–H1927. - PubMed
-
- ADRIE C., ICHINOSE F., HOLZMANN A., KEEFER L., HURFORD W.E., ZAPOL W.M. Pulmonary vasodilation by nitric oxide gas and prodrug aerosols in acute pulmonary hypertension. J. Appl. Physiol. 1998;8:435–441. - PubMed
-
- ALBERT G.P., DAVIS J.M., ROBBINS C.G., STEINHORN R.H. Recombinant human CuZn superoxide dismutase (rhSOD) as an adjunctive therapy for inhaled nitric oxide. Pediatr. Res. 1999;45 Suppl.:293A.
-
- ANGULO J., SANCHEZ-FERRER C.F., PEIRO C., MARÍN J., RODRÍGUEZ-MAÑAS L. Impairment of endothelium-dependent relaxation by increasing percentages of glycosylated human hemoglobin. Possible mechanisms involved. Hypertension. 1996;28:583–592. - PubMed
-
- BARNES P.J., LIU S.F. Regulation of pulmonary vascular tone. Pharmacol. Rev. 1995;47:87–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

