Different fast-gate regulation by external Cl(-) and H(+) of the muscle-type ClC chloride channels
- PMID: 11429442
- PMCID: PMC2233746
- DOI: 10.1085/jgp.118.1.23
Different fast-gate regulation by external Cl(-) and H(+) of the muscle-type ClC chloride channels
Abstract
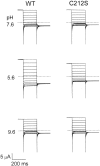
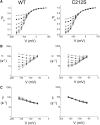
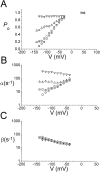
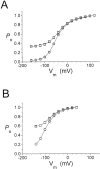
The fast gate of the muscle-type ClC channels (ClC-0 and ClC-1) opens in response to the change of membrane potential (V). This gating process is intimately associated with the binding of external Cl(-) to the channel pore in a way that the occupancy of Cl(-) on the binding site increases the channel's open probability (P(o)). External H(+) also enhances the fast-gate opening in these channels, prompting a hypothesis that protonation of the binding site may increase the Cl(-) binding affinity, and this is possibly the underlying mechanism for the H(+) modulation. However, Cl(-) and H(+), modulate the fast-gate P(o)-V curve in different ways. Varying the external Cl(-) concentrations ([Cl(-)](o)) shifts the P(o)-V curve in parallel along the voltage axis, whereas reducing external pH mainly increases the minimal P(o) of the curve. Furthermore, H(+) modulations at saturating and nonsaturating [Cl(-)](o) are similar. Thus, the H(+) effect on the fast gating appears not to be a consequence of an increase in the Cl(-) binding affinity. We previously found that a hyperpolarization-favored opening process is important to determine the fast-gate P(o) of ClC-0 at very negative voltages. This [Cl(-)](o)-independent mechanism attracted little attention, but it appears to be the opening process that is modulated by external H(+).
Figures